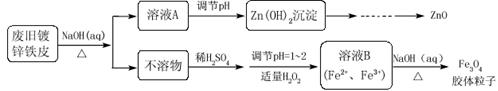
��Ŀ����
ʵ������Ҫ0��2mol/L CuSO4��Һ250ml��ʵ���ҿ��ṩ������Һ���Լ��У�����ɫ��������(CuSO4��5H2O) ��4mol/L CuSO4��Һ
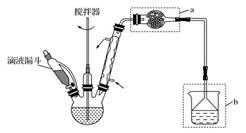
(1)���۲��ú����Լ��������ƣ�ʵ������õ��IJ����������ձ�������������ͷ�ι��⣬���ٻ���Ҫ��һ��������________����ʹ�ø�����ǰ������еIJ����� ��
(2)���õ�������������ƣ�����������ƽ��ȡCuSO4�� 5H2O������Ϊ________g�������4mol/L��CuSO4��Һϡ�����ƣ�������Ͳ��ȡ___________ml4mol/L CuSO4��Һ��
(3)ʵ������4mol/L������ͭ��Һϡ��������Һ�����ʵ�鲽���У�
������ȷ�IJ���˳��Ϊ
�����ձ��м���Լ100mlˮ���г���ϡ�ͣ���ȴ������
������Ͳ��ȡһ�����4mol/L ������ͭ��Һ��һ�ձ���
�ۼ�������4mol/L ����ͭ��Һ�����
�ܽ���Һ�ߵ�ҡ�Ⱥ�ת�����Լ�ƿ
�ݼ�ˮ��Һ��������ƿ1-2cm�����ý�ͷ�ιܽ��ж���
��ϴ���ձ��Ͳ�����2-3�β���ϴ��Һע������ƿ������ҡ������ƿ��ʹ��Һ��Ͼ���
�߽���Һת��������ƿ
(4)������Һ�����У������������������Խ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�������ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶���
B������ʱ���ӿ̶���
C������ƿδ���T����������Һ
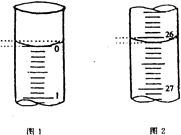
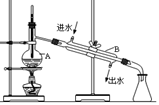
��5��ʵ��������õ�����ˮ����ͼΪʵ������ȡ����ˮ��װ��ʾ��ͼ��ͼ�е��������ԵĴ����� ______________________________��_______________________________��ʵ��ʱA�г�������������ˮ�⣬�����������___ ____���������Ƿ�ֹ���С�
(1)���۲��ú����Լ��������ƣ�ʵ������õ��IJ����������ձ�������������ͷ�ι��⣬���ٻ���Ҫ��һ��������________����ʹ�ø�����ǰ������еIJ����� ��
(2)���õ�������������ƣ�����������ƽ��ȡCuSO4�� 5H2O������Ϊ________g�������4mol/L��CuSO4��Һϡ�����ƣ�������Ͳ��ȡ___________ml4mol/L CuSO4��Һ��
(3)ʵ������4mol/L������ͭ��Һϡ��������Һ�����ʵ�鲽���У�
������ȷ�IJ���˳��Ϊ
�����ձ��м���Լ100mlˮ���г���ϡ�ͣ���ȴ������
������Ͳ��ȡһ�����4mol/L ������ͭ��Һ��һ�ձ���
�ۼ�������4mol/L ����ͭ��Һ�����
�ܽ���Һ�ߵ�ҡ�Ⱥ�ת�����Լ�ƿ
�ݼ�ˮ��Һ��������ƿ1-2cm�����ý�ͷ�ιܽ��ж���
��ϴ���ձ��Ͳ�����2-3�β���ϴ��Һע������ƿ������ҡ������ƿ��ʹ��Һ��Ͼ���
�߽���Һת��������ƿ
(4)������Һ�����У������������������Խ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�������ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶���
B������ʱ���ӿ̶���
C������ƿδ���T����������Һ
��5��ʵ��������õ�����ˮ����ͼΪʵ������ȡ����ˮ��װ��ʾ��ͼ��ͼ�е��������ԵĴ����� ______________________________��_______________________________��ʵ��ʱA�г�������������ˮ�⣬�����������___ ____���������Ƿ�ֹ���С�

��1��250mL����ƿ��1�֣��� ����Ƿ�©Һ��1�֣���
��2��12��5��1�֣��� 12��5��1�֣���
��3��???????��2�֣���
��4��ƫ�ͣ�1�֣��� ƫ�ߣ�1�֣��� ��Ӱ�죨1�֣���
��5���¶ȼƵ�ˮ����Ӧ��������ƿ֧�ܿڴ���1�֣��� ������ӦΪ�¿ڽ�ˮ���Ͽڳ�ˮ����1�֣��� ���Ƭ����ʯ����1�֣���
��2��12��5��1�֣��� 12��5��1�֣���
��3��???????��2�֣���
��4��ƫ�ͣ�1�֣��� ƫ�ߣ�1�֣��� ��Ӱ�죨1�֣���
��5���¶ȼƵ�ˮ����Ӧ��������ƿ֧�ܿڴ���1�֣��� ������ӦΪ�¿ڽ�ˮ���Ͽڳ�ˮ����1�֣��� ���Ƭ����ʯ����1�֣���
���������
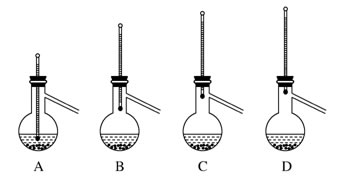
��1��ʵ������Ҫ0��2mol/L CuSO4��Һ250ml��������Ҫ250mL����ƿ��
ʹ�ø�����ǰ������еIJ���Ҫ����Ƿ�©Һ������Ӱ��ʵ�����ݡ�
CuSO4�����ʵ���n=cV=0��25L��0��2mol?L-1=0��05mol��CuSO4?5H2O�����ʵ�������CuSO4�����ʵ���������CuSO4?5H2O������0��05mol��250g/mol=12��5g��
�����4mol/L��CuSO4��Һϡ�����ƣ�������Ͳ��ȡ4mol/L CuSO4��Һ�����Ϊ��0��05mol/(4mol/L )=0��0125L=12��5mL��
��3��???????
��4��A�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ�������Һ������ƿ����ƿ��֮�䣬�ټ�ˮ���̶��ߣ�������Һ�����ƫ����ҺŨ��ƫС��
B������ʱ���ӿ̶��ߣ�����������Һ�����ƫ����ҺŨ��ƫС��
C����Һ�������ˮ���ݣ�����ƿδ���T����������Һ����������ҺŨ����Ӱ�죻
��5���¶ȼƵ�ˮ����Ӧ��������ƿ֧�ܿڴ���������ӦΪ�¿ڽ�ˮ���Ͽڳ�ˮ�� ���Ƭ����ʯ����

��ϰ��ϵ�д�
�����Ŀ