��Ŀ����
5�������йذ����ӵ�����˵����ȷ������ǣ��������ٱ�״���£�22.4L������ȫ��Ӧ��ת�Ƶ�����Ŀһ��Ϊ2NA
��25��ʱ��pH=13��1.0L��Ba��OH��2��Һ�к��е�OH-��ĿΪ0.2NA
�۵�⾫��ͭʱ����������������64g����ת�Ƶ������ĵ�������һ������2NA
�ܱ�״���£�2.24L�����⺬�е�ԭ����Ϊ0.2NA
��2.24LCO2��2.8gN2��ɵĻ������������Ϊ2.8NA
��24g3H218O���е�������Ϊ12NA��
| A�� | �� | B�� | �٢� | C�� | �ۢ� | D�� | �ڢݢ� |
���� ����������ֻ����������Ҳ��������������ԭ��Ӧ��
������pH����������Ũ�ȣ������Һ�����ӻ������������Һ������������Ũ�ȣ�ˮ��Һ��ˮ����Ҳ�������������ӷ����жϣ�
�۸��ݴ�ͭ�к������ʣ�������Ħ��������ͭС��ת�Ƶĵ��ӵ����ʵ�������2mol������
������Ħ�����ֻ�������壻
������״��δ֪�����ʵ��������㣻
��3H218O���е�������Ϊ14��
��� �⣺��1 mol �����μӷ�Ӧʱ����������ֻ����������Ҳ��������������ԭ��Ӧ������ת����Ŀ����Ϊ2NA����NA���ʴ���
��25��ʱ��pH=13��1.0L Ba��OH��2��Һ�У���е�OH-��ĿΪ0.2NA��ˮ���ڵ����������������ӣ�������Һ�����������Ӵ���0.2NA���ʴ���
�۵�⾫��ͭʱ����������������64g�����������������ʴ��ڣ�����Ħ������С��ͭ�ģ�������������64g��ת�Ƶĵ��ӵ����ʵ�������2mol���������õ��ĵ���������2mol������ȷ��
�ܱ���·�����ΪҺ̬������ʹ������Ħ��������ʴ���
��CO2����״��δ֪�����ʵ��������㣬�ʴ���
��24g3H218O�����ʵ���Ϊ$\frac{24}{24}$=1mol���ʺ��е�������Ϊ14mol���ʴ���
��ѡ��A��
���� ���⿼�鰢���ӵ���������ؼ��㣬�������������ʵ���Ϊ���ĵ�ת����ϵ������Ħ�������ʹ�������ǽ���ؼ�����Ŀ�ѶȲ���

 �ο�������ϵ�д�
�ο�������ϵ�д� ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д�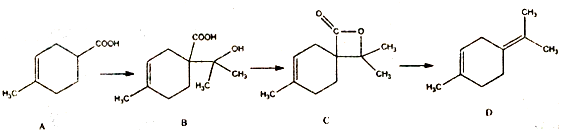
����˵����ȷ���ǣ�������
| A�� | 1mol�л���B�ֱ���������Na��Na2CO3��Һ��Ӧ���ֱ�����lmolH2 ��lmolCO2 | |
| B�� | �л���B�ķ���ʽΪC11H18O3�����ܷ���ȡ�����ӳɺ���ȥ��Ӧ | |
| C�� | �л���c����ͬ���칹���в������з����廯������� | |
| D�� | �л���D����������̼ԭ��һ������ |
�ٵ�ˮ��������Һ ����ˮ��CCl4 ����ˮ���� �����ᡢAgNO3��Һ ����ˮ���� ����ˮ�����ᣮ
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �ڢܢ� | D�� | �ܢݢ� |
| A�� | O3��NO2 | B�� | CH4��NH4+ | C�� | N2O��CO2 | D�� | PCl3��SO32- |
| A�� | HF | B�� | Na2O2 | C�� | CaCl2 | D�� | H2SO4 |

 ��
��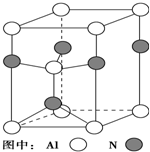 Ԫ�����ڱ��еĵڢ�A����A����A��IJ���Ԫ���������γ�ԭ�Ӿ��壬��������������������黯�����ڴ��У��絪�������徧������ͼ��ʾ
Ԫ�����ڱ��еĵڢ�A����A����A��IJ���Ԫ���������γ�ԭ�Ӿ��壬��������������������黯�����ڴ��У��絪�������徧������ͼ��ʾ
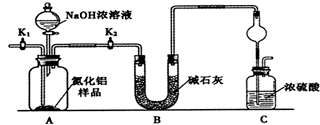
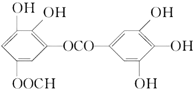 ij�л���Ľṹ��ʽ�ǣ���ͼ�������й�����������������ȷ���ǣ�������
ij�л���Ľṹ��ʽ�ǣ���ͼ�������й�����������������ȷ���ǣ�������