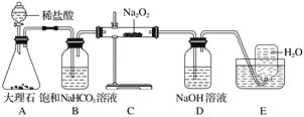
��Ŀ����
����Ŀ����һ�������£��ݻ�Ϊ2 L���ܱ������У���2 mol L�����3 mol M�����ϣ��������·�Ӧ��2L(g)��3M(g)![]() xQ(g)��3R(g)��10sĩ������2.4 mol R�������Q��Ũ��Ϊ0.4 mol��L��1�����㣺
xQ(g)��3R(g)��10sĩ������2.4 mol R�������Q��Ũ��Ϊ0.4 mol��L��1�����㣺
��1��10 sĩL�����ʵ���Ũ��Ϊ_____________��
��2��ǰ10 s����M��ʾ�Ļ�ѧ��Ӧ����Ϊ_____________��
��3����ѧ����ʽ��xֵΪ_____________��
��4���ں��º����������������м���1 mol��������Ӧ����________(����С������)��
��5���ں��º�ѹ�������������м���1 mol��������Ӧ����________(����С������)��
���𰸡���1��0.2mol/L��
��2��0.12mol/(Ls)��
��3��1����4�����䣻��5����С��
�������������������2min��ƽ�⣬����2.4molR�������Q��Ũ��Ϊ0.4mol/L��Q���ʵ���=0.4mol/L��2L=0.8mol�����ʵ���֮�ȵ��ڻ�ѧ������֮�ȣ�0.8��2.4=x��3������õ�x=1��
2L��g��+3M��g��![]() Q��g��+3R��g��
Q��g��+3R��g��
��ʼ����mol�� 2 3 0 0
�仯����mol�� 1.6 2.4 0.8 2.4
10sĩ��mol�� 0.4 0.6 0.8 2.4
��1��10sĩL�����ʵ���Ũ��=![]() =0.2mol/L��
=0.2mol/L��
��2��ǰ10s����M��ʾ�Ļ�ѧ��Ӧ����=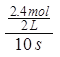 =0.12mol/(Ls)��
=0.12mol/(Ls)��
��3����2min��ƽ�⣬����2.4molR�������Q��Ũ��Ϊ0.4mol/L��Q���ʵ���=0.4mol/L��2L=0.8mol�����ʵ���֮�ȵ��ڻ�ѧ������֮�ȣ�0.8��2.4=x��3������õ�x=1��
��4���ں��º����������������м���1mol��������ѹ����ѹ����ƽ�ⲻ������Ӧ���ʲ��䣻
��5���ں��º�ѹ�������������м���1mol������Ϊ���ֺ�ѹ��������ѹǿ��С����Ӧ���ʼ�С��