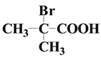ћвƒњƒЏ»Ё
°Њћвƒњ°њЅтїѓЉоЈ® «є§“µ…ѕ÷∆±ЄЅтіъЅтЋбƒ∆ЊІће£®Na2S2O35H2O£©µƒЈљЈ®÷Ѓ“ї£ђЅч≥ћ»зѕ¬£Ї
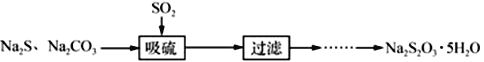
“—÷™£ЇNa2S2O3‘Џњ’∆ш÷–«њ»»їб±ї—хїѓ£ђNa2S2O35H2O£®M=248g/moL£©‘Џ35 °ж“‘…ѕµƒЄ…‘пњ’∆ш÷–“„ І»•љбЊІЋЃ£ђњ…”√„чґ®”∞ЉЅ°Ґїє‘≠ЉЅ°£ƒ≥–Ћ»§–°„й‘Џ µ—й “”√ЅтїѓЉоЈ®÷∆±ЄNa2S2O35H2O≤ҐћљЊњNa2S2O3µƒїѓ—І–‘÷ °£
I£Ѓ÷∆±ЄNa2S2O35H2O
…иЉ∆»зѕ¬ќьЅт„∞÷√£Ї

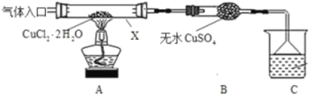
£®1£©–і≥цA∆њ÷–…ъ≥…Na2S2O3ЇЌCO2µƒјл„”Јљ≥ћ љ______°£
£®2£©„∞÷√Bµƒ„ч”√ «Љм—й„∞÷√A÷–SO2µƒќь ’–Ієы£ђ„∞÷√B÷– ‘ЉЅњ…“‘ «______
A ≈®ЅтЋб B деЋЃ C FeSO4»№“Ї D BaCl2»№“Ї
II£Ѓ≤вґ®≤ъ∆Јіњґ»
£®1£©Na2S2O3»№“Ї «ґ®Ѕњ µ—й÷–µƒ≥£”√ ‘ЉЅ£ђ≤вґ®∆д≈®ґ»µƒєэ≥ћ»зѕ¬£Ї
µЏ“ї≤љ£Ї„Љ»Ј≥∆»°a gKIO3£®M=214g/moL£©єћће≈д≥…»№“Ї£ї
µЏґю≤љ£ЇЉ”»лєэЅњKIЇЌH2SO4»№“Ї£ђµќЉ”÷Є ЊЉЅ£ї
µЏ»э≤љ£Ї”√Na2S2O3»№“Їµќґ®÷Ѕ÷’µг£ђѕыЇƒNa2S2O3»№“Їµƒћеїэќ™V mL°£
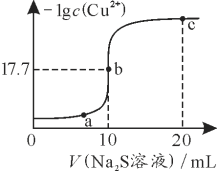
‘тc£®Na2S2O3£©£љ______ mol /L°££®Ѕ–≥цЋг љЉіњ…£©£®“—÷™£ЇIO3£≠+5I£≠+6H+£љ3I2+3H2O£ђ2S2O32£≠+I2£љS4O62£≠+2I£≠£©
£®2£©µќґ®єэ≥ћ÷–ѕ¬Ѕ– µ—й≤ў„чїб‘м≥…љбєы∆ЂЄяµƒ «_________£®ћо„÷ƒЄ£©
A µќґ®є№ќі”√Na2S2O3»№“Ї»уѕі
B µќґ®÷’µг ±Є© ”ґЅ э
C „ґ–ќ∆њ”√’фЅуЋЃ»уѕіЇуќі”√іэ»°“Ї»уѕі
D µќґ®є№Љв„мі¶µќґ®«∞”–∆ш≈Ё£ђіпµќґ®÷’µг ±ќіЈҐѕ÷”–∆ш≈Ё
Ґу£ЃћљЊњNa2S2O3µƒїѓ—І–‘÷
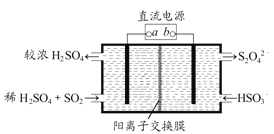
“—÷™Na2S2O3»№“Ї”лCl2Јі”¶ ±£ђ1mol Na2S2O3„™“∆8molµз„”°£Љ„Ќђ—І…иЉ∆»зЌЉ µ—йЅч≥ћ£Ї
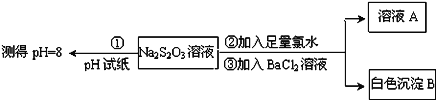
£®1£©Љ„Ќђ—І…иЉ∆ µ—йЅч≥ћµƒƒњµƒ «÷§√чNa2S2O3»№“ЇЊя”–___________ЇЌ__________°£
£®2£©““Ќђ—І»ѕќ™”¶љЂ…ѕ цЅч≥ћ÷–ҐЏҐџЋщЉ” ‘ЉЅЋ≥–тµяµє£ђƒг»ѕќ™јн”… «__________°£
°Њір∞Є°њ2S2£≠+ CO32£≠+ 4SO2 £љ3S2O32£≠+ CO2 B ![]() £®їт
£®їт![]() £© B Љо–‘ їє‘≠–‘ њ…“‘≈≈≥эBaS2O3µƒЄ…»≈
£© B Љо–‘ їє‘≠–‘ њ…“‘≈≈≥эBaS2O3µƒЄ…»≈
°Њљвќц°њ
I£Ѓ(1)ЄщЊЁЌЉ Њ–≈ѕҐњ…÷™£ђќьЅт„∞÷√A÷∆»°Na2S2O3µƒЈі”¶ќпќ™SO2°ҐNa2SЇЌNa2CO3£ђ÷ч≤ъќпќ™Na2S2O3£ђЄщЊЁµ√ Іµз„” э ЎЇгµ√≥цSO2°ҐNa2S°ҐNa2S2O3µƒЉ∆Ѕњ э£ђ‘ўЄщЊЁ÷ Ѕњ ЎЇгµ√≥цNa2CO3µƒЉ∆Ѕњ эЇЌЅн“ї÷÷≤ъќпCO2£ђЊЁіЋЈ÷ќц£ї
(2)ґю—хїѓЅтЊя”–їє‘≠–‘°Ґ∆ѓ∞„–‘£ї
II£Ѓ(1)ЄщЊЁKIO3µƒЅњ«у≥цI2£ђ‘ўЄщЊЁS2O32-”лI2µƒєЎѕµ«у≥цNa2S2O3µƒќп÷ µƒЅњЉ∞≈®ґ»£ї
(2)µќґ® ±µƒќу≤оЈ÷ќц£ђ–ијы”√c(±к)V(±к)=c(іэ)V(іэ)£ђc(іэ)=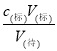 Ј÷ќц£ї
Ј÷ќц£ї
Ґу£Ѓ(1) Љ„Ќђ—Іµƒ µ—йЅч≥ћ÷–Ќ®єэЉ”»лBaCl2 ≤ъ…ъ∞„…Ђ≥ЅµнB јі÷§√чNa2S2O3 ”묻ЋЃЈі”¶ ±”–SO42-…ъ≥…£ї
(2) ‘Џ÷§√чNa2S2O3µƒїє‘≠–‘ ±”…”Џ≤ї÷™µјBaS2O3 «Јс «≥Ѕµн£ђЋщ“‘”¶ѕ»Љ”BaCl2 »№“Ї£ђ»зєы≤ї≤ъ…ъ∞„…Ђ≥Ѕµн‘ўЉ”„гЅњ¬»ЋЃ≤ъ…ъ∞„…Ђ≥Ѕµн£ђЉіњ…÷§√чNa2S2O3Њя”–їє‘≠–‘°£
I£Ѓ(1) ЄщЊЁЌЉ Њ–≈ѕҐњ…÷™£ђќьЅт„∞÷√A÷∆»°Na2S2O3µƒЈі”¶ќпќ™SO2°ҐNa2SЇЌNa2CO3£ђ÷ч≤ъќпќ™Na2S2O3£ђSO2°ҐNa2S÷–Ѕт‘™ЋЎ”…+4ЉџЇЌ2Љџ±дќ™+2Љџ£ђЄщЊЁµ√ Іµз„” э ЎЇгµ√≥цSO2°ҐNa2S°ҐNa2S2O3µƒЉ∆Ѕњ эЈ÷±рќ™4°Ґ2ЇЌ3£ђ‘ўЄщЊЁ÷ Ѕњ ЎЇгµ√≥цNa2CO3µƒЉ∆Ѕњ эќ™1£ђЄщЊЁћЉ‘≠„”ЇЌ—х‘≠„” э ЎЇгњ…÷™Ѕн“ї÷÷≤ъќпCO2£ђ«“Љ∆Ѕњ эќ™1£ђє Јљ≥ћ љќ™£Ї2S2£≠+ CO32£≠+ 4SO2£љ3S2O32£≠+ CO2£ї
(2)ґю—хїѓЅтЊя”–їє‘≠–‘°Ґ∆ѓ∞„–‘£ђЋщ“‘њ…“‘”√∆ЈЇм°ҐдеЋЃїт![]() »№“Ї£ђјіЉм—йґю—хїѓЅт «Јс±їЌк»Ђќь ’£ђ»фSO2ќь ’–І¬ µЌ£ђ‘тґю—хїѓЅт”– £”а£ђB÷–µƒ»№“ЇїбЌ …Ђ£ї
»№“Ї£ђјіЉм—йґю—хїѓЅт «Јс±їЌк»Ђќь ’£ђ»фSO2ќь ’–І¬ µЌ£ђ‘тґю—хїѓЅт”– £”а£ђB÷–µƒ»№“ЇїбЌ …Ђ£ї
II£Ѓ(1) KIO3+5KI+3H2SO4=3K2SO4+3I2+3H2O£ђI2+2Na2S2O3=Na2S4O6+2NaI£ї
n(KIO3)=![]() mol£ђ…и≤ќЉ”Јі”¶µƒNa2S2O3ќ™xmol£ї
mol£ђ…и≤ќЉ”Јі”¶µƒNa2S2O3ќ™xmol£ї

Ћщ“‘x=![]() £ђ‘тc(Na2S2O3)=
£ђ‘тc(Na2S2O3)= =
=![]() molL1£®їт
molL1£®їт![]() £©molL1£ђ
£©molL1£ђ
(2) A.µќґ®є№ƒ©”√Na2S2O3»№“Ї»уѕі£ђ‘тNa2S2O3»№“Їїб±їѕ° Ќ£ђµќґ® ±ѕыЇƒіэ≤в“Їћеїэ∆Ђіу£ђµЉ÷¬іњґ»∆ЂµЌ£ђє A≤їЈыЇѕћв“в£ї
B. µќґ®÷’µг ±Є© ”ґЅ э£ђ єNa2S2O3»№“Їћеїэ∆Ђ–°£ђµќґ® ±ѕыЇƒіэ≤в“Їћеїэ∆Ђ–°£ђµЉ÷¬іњґ»∆ЂЄя£ђє BЈыЇѕћв“в£ї
C.„ґ–ќ∆њ”√’фЅуЋЃ»уѕі£ђґ‘ µ—йљбєы√ї”∞ѕм£ђіњґ»≤ї±д£ђє C≤їЈыЇѕћв“в£ї
D. µќґ®є№Љв„мі¶µќґ®«∞”–∆ш≈Ё£ђіпµќґ®÷’µг ±ќіЈҐѕ÷”–∆ш≈Ё£ђіэ≤в“Їћеїэ∆Ђіу£ђµЉ÷¬—щ∆Јіњґ»∆ЂµЌ£ђє D≤їЈыЇѕћв“в£ї
є ір∞Є—°B£ї
Ґу£Ѓ(1)Љ„Ќђ—ІЌ®єэ≤вґ®Na2S2O3»№“ЇµƒpH=8£їЋµ√чЄ√—ќµƒЋЃ»№“Їѕ‘Љо–‘£їЉ„Ќђ—Іµƒ µ—йЅч≥ћ÷–Ќ®єэЉ”»лBaCl2≤ъ…ъ∞„…Ђ≥ЅµнBјі÷§√чNa2S2O3”묻ЋЃЈі”¶ ±”–SO42…ъ≥…,Љі÷§√чS2O32Њя”–їє‘≠–‘£ї
(2)‘Џ÷§√чNa2S2O3µƒїє‘≠–‘ ±”…”Џ≤ї÷™µјBaS2O3 «Јс «≥Ѕµн£ђЋщ“‘”¶ѕ»Љ”BaCl2»№“Ї£ђ»зєы≤ї≤ъ…ъ∞„…Ђ≥Ѕµн‘ўЉ”„гЅњ¬»ЋЃ≤ъ…ъ∞„…Ђ≥Ѕµн£ђЉіњ…÷§√чNa2S2O3Њя”–їє‘≠–‘£ђє ““њ…≈≈≥эBaS2O3µƒЄ…»≈°£

 ћмћмѕт…ѕ“ї±ЊЇ√ЊнѕµЅ–ір∞Є
ћмћмѕт…ѕ“ї±ЊЇ√ЊнѕµЅ–ір∞Є –°—І…ъ10Ј÷÷””¶”√ћвѕµЅ–ір∞Є
–°—І…ъ10Ј÷÷””¶”√ћвѕµЅ–ір∞Є