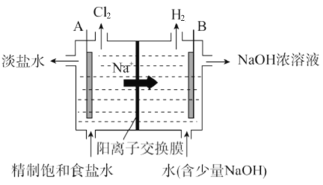
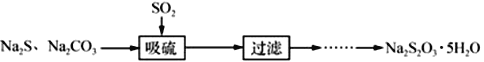
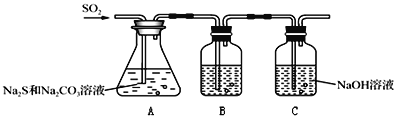
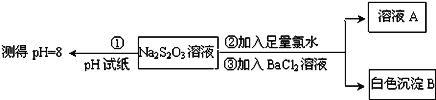
Ő‚ńŅńŕ»›
°ĺŐ‚ńŅ°Ņ25°ś Ī£¨ŌÚ10 mL 0.l mol°§L£≠1 H2C2O4»‹“ļ÷–Ķőľ”Ķ»Ň®∂»ĶńNaOH»‹“ļ£¨»‹“ļĶńpH”ŽNaOH»‹“ļĶńŐŚĽżĻōŌĶ»ÁÕľňý ĺ£¨Ō¬Ń––ū Ų’ż»∑Ķń «( )
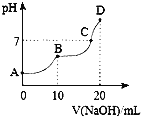
A.AĶ„»‹“ļ÷–£¨c(H£ę)£Ĺc(OH£≠)£ęc(HC2O4£≠)£ę2c(C2O42£≠)
B.HC2O4£≠‘໋“ļ÷–ňģĹ‚≥Ő∂»īů”ŕĶÁņŽ≥Ő∂»
C.CĶ„»‹“ļ÷–ļ¨”–īůŃŅNaHC2O4ļÕH2C2O4
D.DĶ„»‹“ļ÷–£¨c(Na£ę)£ĺc(C2O42£≠)£ĺc(HC2O4£≠)£ĺc(OH£≠)£ĺc(H£ę)
°ĺīūįł°ŅA
°ĺĹ‚őŲ°Ņ
A. AĶ„ «≤›ňŠ»‹“ļ£¨łýĺ›ĶÁļ… ōļ„£¨c(H£ę)£Ĺc(OH£≠)£ęc(HC2O4£≠)£ę2c(C2O42£≠)£¨Ļ A’ż»∑£Ľ
B.BĶ„ «10 mL 0.l mol°§L£≠1 H2C2O4»‹“ļ”Ž10 mL 0.l mol°§L£≠1NaOH»‹“ļĽžļŌ£¨»‹÷ «NaHC2O4£¨BĶ„»‹“ļ≥ ňŠ–‘£¨Ņ…÷™HC2O4£≠‘໋“ļ÷–ĶÁņŽ≥Ő∂»īů”ŕňģĹ‚≥Ő∂»£¨Ļ BīŪőů£Ľ
C. BĶ„»‹÷ «NaHC2O4£¨DĶ„»‹÷ «Na2C2O4£¨ňý“‘CĶ„»‹“ļ÷–ļ¨”–NaHC2O4ļÕNa2C2O4£¨Ļ CīŪőů£Ľ
D. DĶ„»‹“ļĶń»‹÷ «Na2C2O4£¨c(Na£ę)£ĺc(C2O42£≠)£ĺc(OH£≠)£ĺc(HC2O4£≠)£ĺc(H£ę)£¨Ļ DīŪőů°£
īūįł—°A°£

°ĺŐ‚ńŅ°Ņń≥ő¬∂»Ō¬£¨ŌÚ![]() ļ„»›√‹Ī’»›∆ų÷–≥š»Ž
ļ„»›√‹Ī’»›∆ų÷–≥š»Ž![]() ļÕ
ļÕ![]() £¨∑Ę…ķ∑ī”¶
£¨∑Ę…ķ∑ī”¶![]() £¨ĺ≠Ļż“Ľ∂ő Īľšļů∑ī”¶īÔĶĹ∆Ĺļ‚°£∑ī”¶Ļż≥Ő÷–≤‚∂®Ķń≤Ņ∑÷ żĺ›ľŻŌ¬ĪŪ£¨Ō¬Ń–ňĶ∑®’ż»∑Ķń «
£¨ĺ≠Ļż“Ľ∂ő Īľšļů∑ī”¶īÔĶĹ∆Ĺļ‚°£∑ī”¶Ļż≥Ő÷–≤‚∂®Ķń≤Ņ∑÷ żĺ›ľŻŌ¬ĪŪ£¨Ō¬Ń–ňĶ∑®’ż»∑Ķń «![]()
![]()
| 0 | 5 | 15 | 25 | 35 |
|
|
|
|
|
|
A.«į![]() Ķń∆Ĺĺý∑ī”¶ňŔ¬
Ķń∆Ĺĺý∑ī”¶ňŔ¬ ![]()
B.Ī£≥÷∆šňŻŐűľĢ≤ĽĪš£¨…żłŖő¬∂»£¨∆Ĺļ‚ Ī![]() £¨‘Ú∑ī”¶Ķń
£¨‘Ú∑ī”¶Ķń![]()
C.ŌŗÕ¨ő¬∂»Ō¬£¨∆ū ľ ĪŌÚ»›∆ų÷–≥š»Ž![]() £¨īÔĶĹ∆Ĺļ‚ ĪCĶń◊™ĽĮ¬ īů”ŕ
£¨īÔĶĹ∆Ĺļ‚ ĪCĶń◊™ĽĮ¬ īů”ŕ![]()
D.ŌŗÕ¨ő¬∂»Ō¬£¨∆ū ľ ĪŌÚ»›∆ų÷–≥š»Ž![]() °Ę
°Ę![]() ļÕ
ļÕ![]() £¨∑ī”¶īÔĶĹ∆Ĺļ‚«į
£¨∑ī”¶īÔĶĹ∆Ĺļ‚«į![]() ’ż
’ż![]() ńś
ńś![]()