��Ŀ����
����Ŀ�����������һ��������ɫ���������Ʊ�������صĹ����������£�
�ش��������⣺
��1���������NaClO��______���������������ԭ��������������
��2��������ѳ����γ�NaNO3 �⣬���� ____________���ѧʽ����
��3��������������ܽ�ȵIJ�ͬ���еIJ��������ܽ�ȣ�Na2FeO4 _____���>����<����K2FeO4��
��4������ʵ����K2FeO4�IJ���Ϊ__________________��
��5��ȡ����K2FeO4���Թ��У��������ữ����ס�Թܿڣ��۲쵽��Һ����ϸ��С���ݲ�������Һ��ɫ����ȥ������һ�������ǵ�ľ����ľ����ȼ��������Һ�м���KSCN��Һ����Һ��ΪѪ��ɫ���������²�������2.24L,��μӷ�Ӧ��FeO42- ��Ŀ_____________��K2FeO4��Ϊ��ˮ�����ŵ��������������ɱ�����__________________________________��
��6��ijͬѧ�����������ʵ��̽��������ص��ȶ��ԡ�
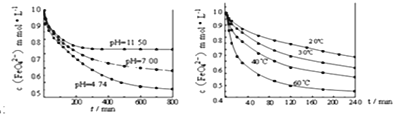
��ʵ��1��������K2FeO4����ֱ��ܽ���pHΪ4.74��7.00��11.50��ˮ��Һ�У����FeO42-Ũ��Ϊ1.0mmolL-1��1mmolL-1=10-3molL-1�������������ã������ͼ1��
��ʵ��2��������K2FeO4�ܽ���pH=4.74��ˮ��Һ�У����Ƴ�FeO42-Ũ��Ϊ1.0mmolL-1���������������ֱ����� 20�桢30�桢40���60��ĺ���ˮԡ�У������ͼ2��
��ʵ����ۣ�����ͼһ�����Եó��Ľ����ǣ�________________________________________
ͼ1 ͼ2
��7�������£�ijˮ��Һ����Fe3+,Cu2+,������ҺpH=10ʱ��������������������棬��֪���¶��£� Ksp��Fe(OH)3��= a, Ksp��Cu(OH)2��= b,����Һ��C(Fe3+)/C(Cu2+)=___________��
���𰸡�������NaCl>86.36%2/15 NA ��2.4��1023���ɵ�Fe(OH)3�������ӿ�����ˮ����������������������ʱ�� pHԽС��K2FeO4Խ���ȶ�104a/b
��������
��1���������Fe3+��NaClO�ڼ�������������ΪFeO42-����NaClO������������ȷ�𰸣���������
(2)�������NaClO�Ļ�ԭ����ΪNaCl����Ӧͬʱ����NaNO3���������ξ���Ҫ��ȥ������Ӱ���Ʒ�Ĵ��ȣ�������ѳ����γ�NaNO3�⣬����NaCl����ȷ�𰸣�NaCl��
(3)�������Na2FeO4��Һ�м���KOH���壬�������ܽ��С��K2FeO4�����ܽ��Na2FeO4>K2FeO4����ȷ�𰸣�>��
(4)4.04gFe(NO3)3��9H2O�����ʵ���Ϊ4.04/404=0.01g��ʵ����K2FeO4�IJ���Ϊ0.01��198/1.71��100%=86.36%����ȷ�𰸣�86.36%��
(5)���������Ϣ��֪��K2FeO4�ữʱ��������Fe3+���ɣ����ݵ����غ㡢����غ㼰ԭ���غ��֪������Ӧ�����ӷ���ʽΪ4FeO42-+20H+=4Fe3++3O2��+10H2O������4FeO42---12e--3O2��ϵ��֪���������²�������2.24L����Ϊ0.1mol��,��μӷ�Ӧ��FeO42- ��Ŀ2/15 NA ��2.4��1023��K2FeO4��Ϊ��ˮ�����ŵ�������⣬��������������ǿ�����ԣ�������ɱ����ͬʱ��ԭ����Fe3+ˮ�����ɵ�Fe(OH)3������ˮ���������ʣ���ȷ�𰸣�2/15 NA ��2.4��1023 �����ɵ�Fe(OH)3�������ӿ�����ˮ���������ʡ�
��6������ͼһ�����Եó��Ľ����ǣ�������������ʱ�� pHԽС��K2FeO4Խ���ȶ� ����ȷ����������������ʱ�� pHԽС��K2FeO4Խ���ȶ���
��7��c(Fe3+)/c(Cu2+)= c(Fe3+)��c3(OH-)/ c(Cu2+)��c2(OH-)��c(OH-)= Ksp[Fe(OH)3]/ Ksp[Cu(OH)2] ��c(OH-)= a/b��c(OH-)��������ҺpH=10ʱ��c(H+)=10-10mol/L������c(OH-)=10-4 mol/L��������ʽ�ɵ�c(Fe3+)/c(Cu2+)= 104a/b����ȷ�𰸣�104a/b��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�������������ʵ���Ҫ���������ʽṹ����ش��������⡣
��1������������һ����������(ֲ���������ڼ�)������ɽṹ���������ʼ��±���
����ʽ | �ṹ��ʽ | ��� | �۵� | �ܽ��� |
C12H10ClN3O |
| ��ɫ�ᾧ��ĩ | 170~172 �� | ������ˮ |
������֪������2-��-4-������������ᱽ����Ӧ�������������塣
![]()
��Ӧ�����У�ÿ����1 mol�������壬����____���Ҽ�������____���м���
��2�����ɽ���������ˮ�����γɵ�������Ƿ�����ɫ����d��������Ų��йء�һ��أ�d0��d10�Ų�����ɫ��d1��d9�Ų�����ɫ����Co(H2O)6]2���Էۺ�ɫ���ݴ��жϣ�Mn(H2O)6]2��_____(��ޡ����С�)��ɫ��
��3����Ԫ�ؾ���ȱ�����ԣ��仯�����������мӺ��ԣ�������ᣨH3BO3����ˮ��Һ������ˮ��Ӧ����B(OH)4]��������һԪ��������ʣ���B(OH)4]����B��ԭ���ӻ�����Ϊ_________��
��4��Mg�ǵ�������Ԫ�أ������ڲ���Ԫ�ط�������۵���±���
������ | NaF | MgF2 | SiF4 |
�۵�/K | 1 266 | 1 534 | 183 |
���ͱ��з������۵�����ԭ��___________________________________��
��5���ҹ���ѧ�ҳɹ��ϳ������������嵪��������(N5)6(H3O)3(NH4)4Cl(��R ����)����X-���������û�����R �ľ���ṹ����ֲ��ṹ����ͼ��ʾ��

����ɻ�����R �������������ЦҼ��ĸ���֮��Ϊ____________�������ĺ������ӵ����幹��Ϊ____________��������ԭ�ӵ��ӻ����������____________��
�ڷ����еĴ�м����÷��ű�ʾ��mn������m ���������γɵĴ�м�ԭ������n ���������γɵĴ�м����������籽�����еĴ�м��ɱ�ʾΪ��66����N5���еĴ�м�Ӧ��ʾΪ____________��
�����ʾ����ͼ�е����:________________________��
