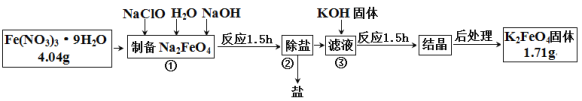
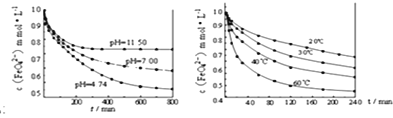
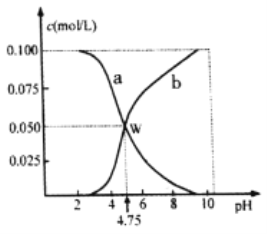
��Ŀ����
����Ŀ�������������ʵ���Ҫ���������ʽṹ����ش��������⡣
��1������������һ����������(ֲ���������ڼ�)������ɽṹ���������ʼ��±���
����ʽ | �ṹ��ʽ | ��� | �۵� | �ܽ��� |
C12H10ClN3O |
| ��ɫ�ᾧ��ĩ | 170~172 �� | ������ˮ |
������֪������2-��-4-������������ᱽ����Ӧ�������������塣
![]()
��Ӧ�����У�ÿ����1 mol�������壬����____���Ҽ�������____���м���
��2�����ɽ���������ˮ�����γɵ�������Ƿ�����ɫ����d��������Ų��йء�һ��أ�d0��d10�Ų�����ɫ��d1��d9�Ų�����ɫ����Co(H2O)6]2���Էۺ�ɫ���ݴ��жϣ�Mn(H2O)6]2��_____(��ޡ����С�)��ɫ��
��3����Ԫ�ؾ���ȱ�����ԣ��仯�����������мӺ��ԣ�������ᣨH3BO3����ˮ��Һ������ˮ��Ӧ����B(OH)4]��������һԪ��������ʣ���B(OH)4]����B��ԭ���ӻ�����Ϊ_________��
��4��Mg�ǵ�������Ԫ�أ������ڲ���Ԫ�ط�������۵���±���
������ | NaF | MgF2 | SiF4 |
�۵�/K | 1 266 | 1 534 | 183 |
���ͱ��з������۵�����ԭ��___________________________________��
��5���ҹ���ѧ�ҳɹ��ϳ������������嵪��������(N5)6(H3O)3(NH4)4Cl(��R ����)����X-���������û�����R �ľ���ṹ����ֲ��ṹ����ͼ��ʾ��

����ɻ�����R �������������ЦҼ��ĸ���֮��Ϊ____________�������ĺ������ӵ����幹��Ϊ____________��������ԭ�ӵ��ӻ����������____________��
�ڷ����еĴ�м����÷��ű�ʾ��mn������m ���������γɵĴ�м�ԭ������n ���������γɵĴ�м����������籽�����еĴ�м��ɱ�ʾΪ��66����N5���еĴ�м�Ӧ��ʾΪ____________��
�����ʾ����ͼ�е����:________________________��
���𰸡� NA NA �� sp3 NaF��MgF2Ϊ���Ӿ��壬SiF4Ϊ���Ӿ��壬����NaF��MgF2Զ��SiF4�۵�Ҫ�ߡ���ΪMg2+�İ뾶С��Na+�İ뾶��������������࣬����MgF2�ľ����ܴ���NaF�ľ����ܣ���MgF2���۵����NaF 3:4(��4:3) ������ sp3 ��56 N-H������N�� O-H������N�� N-H������Cl
��������(1).�����ᱽ�����ѵ�N=C�к�1���м���2-��-4-����श��ѵ���1���Ҽ���(2).����������Ϣ��Mn(H2O)6]2����Mnԭ��d�ܼ��������ж������Ƿ�����ɫ��(3). ���ݼ۲���ӶԻ�������ȷ�����ӻ���ʽ��(4).��������۵�ߵ��뾧�������йأ����Ӿ�����۵�ϸߣ����Ӿ�����۵�ϵ���(5). ��.������ΪH3O����NH4����H3O���к���3���Ҽ���NH4���к���4���Ҽ�����. N5���л�ѧ������Ϊ5������6�������γɴ���������. ��ͼ��֪����ɱ�ʾΪN-H������N��O-H������N��N-H������Cl��
(1).�ڷ�Ӧ�����У������ᱽ�����ѵ�N=C�к�1���м���2-��-4-����श��ѵ���1���Ҽ�����ÿ����1 mol�������壬����NA���Ҽ���NA���м����ʴ�Ϊ��NA��NA��
(2). ���ɽ���������ˮ�����γɵ�������Ƿ�����ɫ����d��������Ų��й���һ��أ�d0��d10�Ų�����ɫ��d1��d9�Ų�����ɫ��Mn(H2O)6]2����Mnԭ��d�ܼ�������Ϊ5�����Ը���������ɫ���ʴ�Ϊ���У�
(3). B(OH)4]����B�ļ۲���Ӷ���Ϊ4+![]() =4������Bԭ�ӵ��ӻ�����Ϊsp3���ʴ�Ϊ��sp3��
=4������Bԭ�ӵ��ӻ�����Ϊsp3���ʴ�Ϊ��sp3��
(4). ���Ӿ�����۵�ϸߣ����Ӿ�����۵�ϵͣ�NaF��MgF2Ϊ���Ӿ��壬SiF4Ϊ���Ӿ��壬����NaF��MgF2Զ��SiF4�۵�Ҫ�ߣ�����ΪMg2+�İ뾶С��Na+�İ뾶����Mg2+�ĵ�����࣬����MgF2�ľ����ܴ���NaF������MaF2���۵����NaF���ʴ�Ϊ��NaF��MgF2Ϊ���Ӿ��壬SiF4Ϊ���Ӿ��壬����NaF��MgF2Զ��SiF4�۵�Ҫ�ߡ���ΪMg2+�İ뾶С��Na+�İ뾶��������������࣬����MgF2�ľ����ܴ���NaF�ľ����ܣ���MgF2���۵����NaF��
(5).��.��ͼ��֪��������ΪH3O����NH4����H3O���к���3���Ҽ���NH4���к���4���Ҽ�������R �������������ЦҼ��ĸ���֮��Ϊ3:4(��4:3)��H3O��������ԭ����O����۲���Ӷ���Ϊ3+![]() =3+1=4������Oԭ�ӵ��ӻ�����Ϊsp3���µ��Ӷ���Ϊ1������ռ乹��Ϊ�����Σ��ʴ�Ϊ��3:4(��4:3)����������sp3��
=3+1=4������Oԭ�ӵ��ӻ�����Ϊsp3���µ��Ӷ���Ϊ1������ռ乹��Ϊ�����Σ��ʴ�Ϊ��3:4(��4:3)����������sp3��
��.��ͼ��֪��N5���л�ѧ������Ϊ5������6�������γɴ����������÷�����56��ʾ���ʴ�Ϊ����56��
��.��ͼ��֪����ͼ�е�����ɱ�ʾΪN-H������N��O-H������N��N-H������Cl���ʴ�Ϊ��N-H������N��O-H������N��N-H������Cl��

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�����Ŀ����80 ��ʱ����0.20 mol��N2O4�������1 L�ѳ�յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
ʱ�� /(s) | 0 | 20 | 40 | 60 | 80 | 100 |
c(N2O4) / mol �� L��1 | 0.20 | c1 | 0.10 | c2 | c3 | c4 |
c(NO2) / mol �� L��1 | 0.00 | 0.12 | 0.20 | 0.22 | 0.22 | 0.22 |
���ݱ������ݺͱ����ṩ�������(c1��c2��c3��c4��ʾ��Ӧ��Ũ��)����ش����и�С�⣺
(1)�÷�Ӧ��ѧ����ʽ_________________________�� ����c2____c3(������������������������)
(2)c4��_______mol �� L��1����0��20 s��NO2��ƽ����Ӧ����Ϊ______________________