��Ŀ����
����Ŀ������ԭCO2�ǽ������ЧӦ����Դ�������Ҫ�ֶ�֮һ���о���������Cu/ZnO������������CO2��H2�ɷ�������ƽ�з�Ӧ���ֱ�����CH3OH��CO����Ӧ���Ȼ�ѧ����ʽ����:
I.CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H1=-53.7kJ��mol-1
CH3OH(g)+H2O(g) ��H1=-53.7kJ��mol-1
I.CO2(g)+H2(g) ![]() CO(g)+H2O(g) ��H2
CO(g)+H2O(g) ��H2
ijʵ���ҿ���CO2��H2��ʼͶ�ϱ�Ϊ1:2.2������ͬѹǿ�£�������ͬ��Ӧʱ��������ʵ������:
T(K) | ���� | CO2ת����(��) | �״�ѡ����(��) |
543 | Cat.l | 12.3 | 42.3 |
543 | Cat.2 | 10.9 | 72.7 |
553 | Cat.l | 15.3 | 39.1 |
553 | Cat.2 | 12.0 | 71.6 |
[��ע]XCat.1:Cu/ZnO���װ���Cat.2:Cu/ZnO����Ƭ���״�ѡ����:ת����CO2�����ɼ״��İٷֱȡ�
��֪:��CO��H2�ı�ȼ���ȷֱ�Ϊ-283.0kJ/mol��һ285.8kJ/mol��
��H2O(l)==H2O(g) ��H3=+44.0kJ��mol-1
��ش���������(�������¶ȶ���H��Ӱ��):
��1����ӦI��_________(��������������������)���Է���������ӦIIÿ����9gˮ�����ų�����Ϊ___________��
��2�����������£����������CO2ת��ΪCH3OH��ƽ��ת���ʵĴ�ʩ��_____(����ĸ����)��
A.�ӳ���Ӧʱ�� B.ʹ�ô���Cat.2 C.���ͷ�Ӧ�¶�
D.Ͷ�ϱȲ��䣬���ӷ�Ӧ���Ũ�� E.����CO2��H2�ij�ʼͶ�ϱ�
��3���ɱ���ʵ�����ݿ��Եó��Ľ�����_______________________��
��4��553K��ʹ�ô���Cat.2���ڸ�ʱ��H2��ת����Ϊ______(����ĸ����)��
A.5.5% B.13.3% C.16.4% D.29.3%
��5������ͼ�зֱ���ӦI����������Cat.1����Cat.2�������������Ӧ������������ʾ��ͼ��________
��6���о�֤ʵ��CO2Ҳ����������Һ�������Ե�����ɼ״��������ɼ״��ĵ缫��Ӧʽ��_________����һ���ĵ�����Ϊ______________________��
���𰸡� ���� 20.6kJ CD ����ͬ�¶��²�ͬ�Ĵ�����CO2 ת����CH3OH��ѡ������������Ӱ��(������ͬ�¶��²�ͬ�Ĵ����Է�ӦI�Ĵ�������ͬ) C  CO2+6H++6e-==CH3OH+H2O O2(H2SO4)
CO2+6H++6e-==CH3OH+H2O O2(H2SO4)
����������1����ӦIΪI.CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H1=-53.7kJ��mol-1 Ϊ���ȷ�Ӧ��
CH3OH(g)+H2O(g) ��H1=-53.7kJ��mol-1 Ϊ���ȷ�Ӧ��
�ڵ������Է���������ӦΪII.CO2(g)+H2(g) ![]() CO(g)+H2O(g) ��H2
CO(g)+H2O(g) ��H2
��֪CO��H2��ȼ���ȷֱ�Ϊ-283.0kJ��mol-1��-285.8kJ��mol-1�����٣�CO��g��+![]() O2��g��=CO2��g����H= -283.0kJ��mol-1�ڣ�H2��g��+
O2��g��=CO2��g����H= -283.0kJ��mol-1�ڣ�H2��g��+![]() O2��g��=H2O ��l����H=-285.8kJ��mol-1�ۣ�H2O��l����H2O��g����H3=+44.0kJ��mol-1
O2��g��=H2O ��l����H=-285.8kJ��mol-1�ۣ�H2O��l����H2O��g����H3=+44.0kJ��mol-1
���ݸ�˹���ɷ������ڣ��٣������ɵ��Ȼ�ѧ����ʽΪ��
CO2��g��+ H2��g��![]() CO��g��+H2O��g����H2=-285.8+283.0+44=+41.2 kJ��mo
CO��g��+H2O��g����H2=-285.8+283.0+44=+41.2 kJ��mo
ÿ����9gˮ�����ų�����Ϊ20.6 kJ��
��2����Ӧ����A���ӳ���Ӧʱ�䣬ƽ�ⲻ�ƶ����������ת���ʣ�A����B��ʹ�ô�����ƽ�ⲻ�ƶ����������ת���ʣ�B����C�����ͷ�Ӧ�¶ȣ�ƽ�������ƶ�����߶�����̼��ת���ʣ�C��ȷ��D��Ͷ�ϱȲ��䣬���ӷ�Ӧ��Ũ�ȣ�ƽ�������ƶ�����߶�����̼��ת���ʣ�D��ȷ;E�����������̼�������ij�ʼͶ�ϱȣ������������ת���ʣ�������̼�Ļή�ͣ�E������ѡCD��
��3���ӱ������ݷ���������ͬ���¶��£���ͬ�Ĵ������������̼��ת����Ҳ��ͬ��˵����ͬ�Ĵ����Ĵ�������ͬ����ͬ������ͬ���¶ȣ�������̼��ת���ʲ�ͬ�����¶ȸߵ�ת���ʴ���Ϊ����ӦΪ���ȷ�Ӧ��˵������������δ��ƽ�����ݡ���������ͬ�¶��²�ͬ�Ĵ�����CO2ת����CH3OH��ѡ������������Ӱ����
��4����.CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H1= -53.7kJ��mol-1
CH3OH(g)+H2O(g) ��H1= -53.7kJ��mol-1
��ʼ�� 1 2.2 0 0
�仯��0,12 0.36 0.12 0.12
ƽ���� 0,88 1.84 0.12 0.12
553K��ʹ�ô���Cat.2���ڸ�ʱ��H2��ת����Ϊ=0.36/2.2![]() =16.4
=16.4![]()
��5��֪��ӦI����������Cat.1����Cat.2��������¡���Ӧ������������ʾ��ͼ��_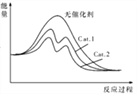
��6��CO2Ҳ��������ˮ��Һ��ͨ��������ɼ״�CԪ�ػ��ϼ۽���,����ԭ,ӦΪ���ص�������Ӧ,�缫����ʽΪCO2+6H++6e- = CH3OH+H2O��������4OH��4e-=O2![]() +H2O
+H2O
�ʴ�Ϊ��CO2+6H++6e- = CH3OH+H2O��O2 (H2SO4)
�㾦:���ո�˹�����Ƿ����ͼ��㷴Ӧ�ȳ��õĹ��ߡ����ݷ���ʽ�ļӼ�ȷ����Ӧ�ȵļӼ���ͬʱע�ⷴӦ�ȵ������š�����ƽ���ƶ�ԭ����ע��ֻ��Ũ�ȡ��¶Ⱥ�ѹǿӲ��ƽ�⣬ע�������ʹ��ֻ�ܸı䷴Ӧ���ʵ���Ӱ��ƽ�⣬����Ӱ�쵽��ƽ���ʱ�䡣������һ�ַ�Ӧ���Ũ��ʱ��ƽ�������ƶ�����һ�ַ�Ӧ���ת���ʻ���ߣ�������ת���ʻή�͡����⿼����ƽ�ⳣ����˹���ɣ�ƽ���ƶ���Ӱ�����أ��ʹ����Է�Ӧ��Ӱ�죬�ۺ�������ǿ����Ŀ�Ѷ��еȡ�

����Ŀ������������ͼʾ�ó��Ľ��۲��������ǣ�������
A | B | C | D | |
ͼʾ |
|
|
|
|
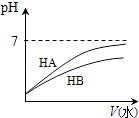
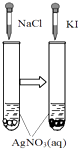
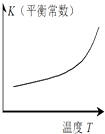
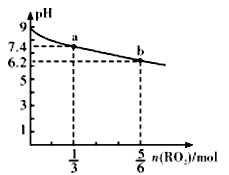
���� | HBΪ���� | HFΪ������� | �ܽ�ȣ�AgI��AgCl | ����Ӧ��H��0 |
A.A
B.B
C.C
D.D