��Ŀ����
15�����м������ʵ��۵㣨�棩���ݣ�| A�� | B�� | C�� | D�� |
| ���ʯ��3550 | Li��181 | HF��-83 | NaCl��801 |
| �辧�壺1410 | Na��98 | HCl��-115 | KCl��776 |
| ���壺2300 | K��64 | HBr��-89 | RbCl��718 |
| �������裺1710 | Rb��39 | HI��-51 | CsCl��645 |
��1��A������ԭ�Ӿ��壬���ۻ�ʱ�˷������Ӽ���������ǻ�ѧ����
��2��B�龧�干ͬ�����������Ǣڢۢܣ�����ţ���
��Ӳ�ȴ� ���ڵ����ԡ� �۵����ԡ� ����չ��
��3��D�龧����ܾ��е������Ǣڢܣ�����ţ���
��Ӳ��С����ˮ��Һ�ܵ��硡�۹����ܵ��� ������״̬�ܵ���
��4��D�龧����۵��ɸߵ��͵�˳��Ϊ��NaCl��KCl��RbCl��CsCl����ԭ�����Ϊ����Ϊ���Ӿ��壬r��Na+����r��K+����r��Rb+����r��Cs+�������������������ͬ������£��뾶ԽС��������Խ���۵��Խ�ߣ�
���� ��1��ֱ����ԭ�ӹ��ɵľ�����ԭ�Ӿ��壬ԭ�Ӿ�������ʱ�ƻ���ѧ����
��2��B�龧�嶼�ǽ������壬���н���ͨ�ԣ�
��3��D�龧���������Ӿ��壬���Ӿ�������̬�ܵ��磬���������Ӿ�����ˮ��Һ���ܵ��磻
��4�����Ӿ�����۷е��뾧���ܳ����ȣ������������Ӱ뾶�ɷ��ȡ����ɳ����ȣ�
��� �⣺��1��ֱ����ԭ�ӹ��ɵľ�����ԭ�Ӿ��壬���龧�嶼��ֱ����ԭ�ӹ��ɵģ�����ԭ�Ӿ��壻ԭ�Ӿ�������ʱ�ƻ���ѧ�����������ۻ�ʱ�˷������Ӽ��������ǻ�ѧ�����ʴ�Ϊ��ԭ�ӣ���ѧ����
��2��B�龧�嶼�ǽ������壬���н���ͨ�ԣ����Ծ��е����ԡ������ԡ���չ�Ե��������ʣ���ѡ�ڢۢܣ�
��3��D�龧���������Ӿ��壬���Ӿ�������̬�ܵ��磬���������Ӿ�����ˮ��Һ���ܵ��磬��̬ʱû�������ƶ������ӣ����Թ��岻���磬��ѡ�ڢܣ�
��4��D�龧�嶼Ϊ���Ӿ��壬r��Na+����r��K+����r��Rb+����r��Cs+�������������������ͬ������£��뾶ԽС��������Խ���۵��Խ�ߣ��ʴ�Ϊ����Ϊ���Ӿ��壬r��Na+����r��K+����r��Rb+����r��Cs+�������������������ͬ������£��뾶ԽС��������Խ���۵��Խ�ߣ�
���� ���⿼�龧������ʣ����ؿ���ԭ�Ӿ��塢���Ӿ��塢������������ʣ�֪�������������Ӿ����۷е��ϵ��ע�����Ӿ��岻һ�����磬Ϊ�״��㣮

 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�| A�� |  | B�� | 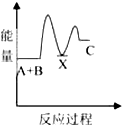 | C�� | 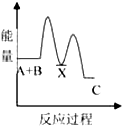 | D�� | 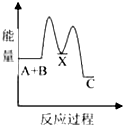 |
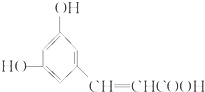
| A�� | X������������9��̼ԭ�ӹ�ƽ�� | |
| B�� | X���Է���ȡ�����ӳɡ���ȥ���ۺϵȷ�Ӧ | |
| C�� | 1 mol X������NaHCO3��Һ��Ӧ�������ʵķ���ʽΪC9H7O4Na | |
| D�� | �����ϵ�ȡ������X��ȫ��ͬ��X��ͬ���칹����5�� |
| A�� | Ũ���� | B�� | ϡ���� | C�� | Ũ���� | D�� | ϡ���� |
| Ԫ�� | I1 | I2 | I3 | I4 |
| X | 500 | 4600 | 6900 | 9500 |
| Y | 580 | 1800 | 2700 | 11600 |
| A�� | Ԫ��X�ij������ϼ���+1 | |
| B�� | Ԫ��X�����γɻ�����ʱ����ѧʽ������XCl | |
| C�� | Ԫ��Y�Ǣ�A��Ԫ�� | |
| D�� | ��Ԫ��Y���ڵ������ڣ���������ˮ���ҷ�Ӧ |
| A�� | ԭ�Ӱ뾶B��C��A | B�� | ԭ������A��B��C | ||
| C�� | ���Ӱ뾶B2-��C-��A+ | D�� | ԭ�ӵĵ�������C��A��B |
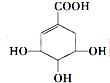
| A�� | ����ʽΪC7H6O5 | |
| B�� | �����к��еĹ��������ǻ���������̼̼˫�� | |
| C�� | �ɷ����ӳɷ�Ӧ��ȡ����Ӧ��������Ӧ����ԭ��Ӧ | |
| D�� | ��ˮ��Һ���Ȼ����ǻ����ܵ����H+��1mol��ƿ���4mol Na��Ӧ����2 mol H2 |
| A�� | ����������䣬����H2ʹ��ϵѹǿ���� | |
| B�� | �����������Сһ�� | |
| C�� | ����C���� | |
| D�� | ����ѹǿ���䣬����N2ʹ��ϵ������� |
 ������������������ʹ���ã��ǽ���ʹ�õĽ�����ʹҩ���С����ȱ���֮�ƣ����Ʊ�ԭ�����£�
������������������ʹ���ã��ǽ���ʹ�õĽ�����ʹҩ���С����ȱ���֮�ƣ����Ʊ�ԭ�����£�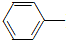 NH2+CH3COOH$\stackrel{��}{��}$
NH2+CH3COOH$\stackrel{��}{��}$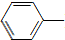 NHCOCH3+H2O
NHCOCH3+H2O��֪��
�ٱ����ױ�������
�����������������ʹ���IJ��������������±���
| ���� | �۵� | �е� | �ܽ�ȣ�20�棩 |
| �������� | 114.3�� | 305�� | 0.46 |
| ���� | -6�� | 184.4 | 3.4 |
| ���� | 16.6�� | 118�� | ���� |
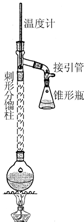
����1����50mLԲ����ƿ�У�����5mL������7.5mL�����ἰ����п�ۣ�������ͼװ����װ������
����2�������¶ȼ�ʾ��Լ105�棬С����Ȼ���1h��
����3�����Ƚ���Ӧ����ﵹ��ʢ��100mL��ˮ���ձ��У���ȴ����ˣ�ϴ�ӣ��õ��ֲ�Ʒ��
��ش��������⣺
��1������1�м���п�۵������Ƿ�ֹ������������ͬʱ���ŷ�ʯ�����ã�
��2������2�п����¶ȼ�ʾ��Լ105���ԭ�����¶ȹ��ߣ�δ��Ӧ���������������ͷ�Ӧ��������ʣ��¶ȹ��ͣ��ֲ��ܳ�ȥ��Ӧ���ɵ�ˮ��
��3������3�г��Ƚ�����ﵹ��ʢ����ˮ���ձ��У������ȡ���ԭ�������÷�Ӧ�������ȴ�����������մ��ƿ���ϲ��״���������װ������������������ѹϵͳ�������ƿ������©�������������ƣ���
��4������3�õ��Ĵֲ�Ʒ���һ���ᴿ�����ᴿ�������ؽᾧ��