��Ŀ����
����Ŀ��ij������Ʒ�к�������NaHCO3���ʣ�������ͼ��ʾװ�����ⶨ������Ʒ��Na2CO3����������(����̨�����е���ͼ�о�����ȥ)��ʵ�鲽�����£�
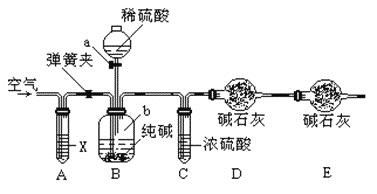
�ٰ�ͼ����װ�ã�����������ԣ�
��ȷ�Ƶ�ʢ�м�ʯ�ҵĸ����D������Ϊm1g��
��ȷ�Ƶ�m2g������Ʒ��������b�У�
�ܴ�����a����������������ϡ���ᣬ�����ٲ�������Ϊֹ��
�ݴ��ɼУ����Թ�A������������������ӣ�Ȼ��Ƶø����D��������Ϊm3g ��
�����������ݼ��㡣�Իش�
��1������a��������___________
��2��װ��B�з�����Ӧ�����ӷ���ʽ_____________________________________��
��3������ݹ��������Ŀ����__________________________________________________��
��4��װ��A���Լ�XӦѡ��______
A ��NaOH B��ŨH2SO4 C��NaHCO3 D��NaCl
��5�������a�����ỻ��Ũ����ͬ�����ᣬ��ᵼ�²ⶨ���______(��ƫ��������ƫС����������)
��6�������������Ʒ�⣬û�������Լ��������һ��Ҳ�ܲⶨ������Ʒ��Na2CO3������������ʵ�鷽������Ҫ���裨����Ҫ���������_________________________�йػ�ѧ����ʽ_____________________��
���𰸡���Һ©�� CO32- + 2H+ = H2O + CO2�� ʹ���ɵ�CO2����ȫ��D���� A ƫ�� ������Ʒ����������ּ��ȣ���ȴ���ڳ���ʣ����������� 2NaHCO3 ![]() Na2CO3 + H2O + CO2��
Na2CO3 + H2O + CO2��
��������
��ʵ����ͨ��̼���ƺ�ϡ���ᷴӦ����������̼���ü�ʯ�������ն�����̼�����ݲ���������̼������������̼���Ƶ���������������������������ʵ�������Ӧ��ֹ������ˮ�Ͷ�����̼����ʵ�飬�Ѳ����Ķ�����̼ȫ������ʯ�����ա�
��1�����������Ľṹ����;������a������Ϊ��Һ©�����ʴ�Ϊ����Һ©����
��2���������εķ�Ӧ���ɣ���Ӧ�Ļ�ѧ����ʽ��Na2CO3+H2SO4�TNa2SO4+H2O+CO2�������ӷ���ʽΪ��CO32-+2H+�TH2O+CO2����
�ʴ�Ϊ��CO32-+2H+�TH2O+CO2����
��3���������֪������ͨ���ⶨ������̼���������ⶨ̼���Ƶ����������ģ�����Ҫʹ���ɵ�CO2����ȫ��D���գ��ʴ�Ϊ��ʹ���ɵ�CO2����ȫ��D���ա�
��4����A��װ�˼�����Һ�����տ����еĶ�����̼����װ��A���Լ���ѡ��NaOH���ʴ�Ϊ��A��
��5����ϡ����Ũ����ͬ��������к�ǿ�Ļӷ��ԣ��ӷ�������HCl�����ܱ���ʯ�������գ���˻�ʹ��ʯ����������ֵƫ��ʹ�ⶨ���ƫ�ߣ��ʴ�Ϊ��ƫ��
��6�������������Ʒ�⣬û�������Լ�������ͨ�����ȳ������������ķ������ⶨ������Ʒ��Na2CO3���������������岽��Ϊ��������Ʒ����������ּ��ȣ���ȴ���ڳ���ʣ������������漰�Ļ�ѧ����ʽΪ��2NaHCO3![]() Na2CO3 +H2O+CO2�����ʴ�Ϊ��������Ʒ����������ּ��ȣ���ȴ���ڳ���ʣ�����������2NaHCO3
Na2CO3 +H2O+CO2�����ʴ�Ϊ��������Ʒ����������ּ��ȣ���ȴ���ڳ���ʣ�����������2NaHCO3![]() Na2CO3 +H2O+CO2����
Na2CO3 +H2O+CO2����

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����ʽ̼��þ[MgCO3��Mg(OH)2��3H2O]����Ҫ�Ļ���ԭ�ϡ������ʯ��ȡ��ʽ̼��þ��һ�ֹ���������ͼ��ʾ��

��֪:������������������ʽ��ʼ������ǡ����ȫ����(������Ũ�ȵ���10-5mol/L)ʱ��pH���±���ʾ��
������ | ��ʼ����ʱ��pH | ������ȫ����ʱ��pH |
Fe3+ | 2.7 | 3.7 |
Mg2+ | 9.7 | 11 |
��1����Mg9FeSi5O20��д�����������ʽ:___________________��
��2�������ʯ�����Ŀ����___________________________��
��3��������pH��ʱ��Ӧ���ڵ�pH��Χ��________________________��
��4������H2O2������Ӧ�����ӷ���ʽΪ_____________________________________��
��5��Ksp[Mg(OH)2]=_______��д�����ɼ�ʽ̼��þ�����ӷ���ʽ��_____________________��