��Ŀ����
����Ŀ������3.60gNaCl��NaHCO3��Na2CO3�Ļ�Ϲ��壬�����㹻��ʱ���������ʣ��3.29g����ʣ���������һ������������У�����0.448L����(��״����)������������Һϡ����100mL�����������ҺpH=1�������ж���ȷ���ǣ� ��
A.��Ϲ�����NaHCO3������Ϊ0.84g
B.��Ϲ�����Na2CO3������Ϊ2.12g
C.���������У�HCl�����ʵ���Ϊ0.04mol
D.����������Һ��c(Cl-)=0.1mol��L-1
���𰸡�A
��������
A��3.60gNaCl��NaHCO3��Na2CO3�Ļ�Ϲ��壬�����㹻��ʱ���������ʣ��3.29g��������3.60g3.29g=0.31g�����ٵ�0.31gΪ̼�����Ʒֽ����£���
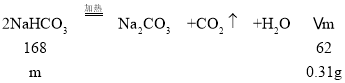
m=![]() =0.84g����A��ȷ��
=0.84g����A��ȷ��
B�������0.448L������̼�����ʵ���Ϊ��![]() =0.02mol������Cԭ���غ��֪��ʣ������к���Na2CO3�����ʵ���Ϊ0.02mol��0.84gNaHCO3�����ʵ���Ϊ��
=0.02mol������Cԭ���غ��֪��ʣ������к���Na2CO3�����ʵ���Ϊ0.02mol��0.84gNaHCO3�����ʵ���Ϊ��![]() =0.01mol�����Cԭ���غ��֪��ԭ�������Na2CO3�����ʵ���Ϊ0.01mol������Ϊ��106g/mol��0.01mol=1.06g����B����
=0.01mol�����Cԭ���غ��֪��ԭ�������Na2CO3�����ʵ���Ϊ0.01mol������Ϊ��106g/mol��0.01mol=1.06g����B����
C�����ݹ�ϵʽNa2CO32HCl��֪������0.02mol������̼����HCl�����ʵ���Ϊ��0.02mol��2=0.04mol����Ӧ��HCl��ʣ�࣬������������HCl�����ʵ�������0.04mol����C����
D��������ҺpH=1��c(HCl)=c(H+)=0.1mol/L��������Һ�л�����NaCl������������Һ��c(Cl)����0.1molL1����D����
��ѡA��

����Ŀ��ij�����̿����Ҫ�ɷ�ΪMnCO3��������������FeCO3��CaCO3��Al2O3�����ʡ���ҵ�������̿�Ϊԭ���Ʊ��ߴ���̼���̵�������ͼ��ʾ��
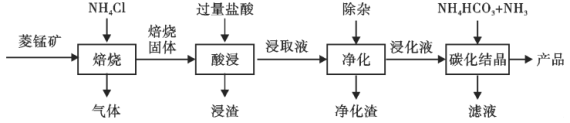
��֪��MnCO3+2NH4Cl=MnCl2+CO2��+2NH3��+H2O��
��ؽ�������[c0��Mn+��=0.1mol��L��1]�γ��������������pH��Χ���£�
�������� | Mn2+ | Fe2+ | Fe3+ | Al3+ |
��ʼ������pH | 8.1 | 6.3 | 1.5 | 3.4 |
������ȫ��pH | 10.1 | 8.3 | 2.8 | 4.7 |
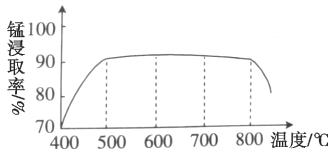
��1������ʱ�¶ȶ��̽�ȡ�ʵ�Ӱ����ͼ������ʱ���˵��¶�Ϊ___________���ң�800�������̵Ľ�ȡ��ƫ�ͣ����ܵ�ԭ����___________��
��2�����������������ټ�������MnO2����������MnO2��������____________��������Ӧ�����ӷ���ʽΪ___________��
�ڼӰ�ˮ��pH����Һ��pH��ΧӦ����Ϊ___________��8.1֮�䡣���ɵij�����Ҫ��___________��
�ۼ���MnF2��������ȥCa2+������Һ��ȹ��ߣ�Ca2+��������ȫ��ԭ����___________��
��3��̼���ᾧ������MnCO3�����ӷ���ʽΪ____________��