��Ŀ����
����Ŀ��������������־����־�����������й��ڰ�ͭ�ļ��أ���������ͭ��ͭ���Ͻ��������⣬����Ҫ������ң������������������Ʒ���ش��������⣺
��1����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ__��3d�ܼ��ϵ�δ�ɶԵĵ�����Ϊ__��
��2�����������ڰ�ˮ�γ�[Ni(NH3)6]SO4��ɫ��Һ��
��[Ni(NH3)6]SO4�������ӵ����幹����__��
����[Ni(NH3)6]2+��Ni2+��NH3֮���γɵĻ�ѧ����Ϊ__���ṩ�µ��ӶԵijɼ�ԭ����__��
�۰��ķе�___������������������������좣�PH3����ԭ����__������__���ӣ����������������Ǽ�������������ԭ�ӵĹ���ӻ�����Ϊ__��
��3������ͭ����������__���γɵľ��壺Ԫ��ͭ�����ĵڶ������ֱܷ�Ϊ��ICu=1959kJ/mol��INi=1753kJ/mol��ICu>INi��ԭ����__��
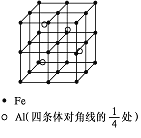
��4��ij����ͭ�Ͻ�����������ṹ��ͼ��ʾ��
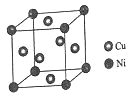
�پ�����ͭԭ������ԭ�ӵ�������Ϊ__��
�����Ͻ���ܶ�Ϊdg/cm3����������a=__nm��
���𰸡�1s22s22p63s23p63d84s2��3d84s2 2 �������� ��λ�� N ���� NH3���Ӽ���γ���� ���� sp3 ���� ͭʧȥ����ȫ������3d10���ӣ���ʧȥ����4s1���� 3��1 ![]() ��107
��107
��������
��1������28��Ԫ�أ�λ�ڵ������ڢ��壬���ݺ�������Ų��������̬ԭ�ӵĵ����Ų�ʽΪ1s22s2 2p63s23p63d84s2��3d�ܼ���5���������ռ��5������������ͬ�ĵ��ӣ�ʣ��3�������ٷֱ�ռ������������������������෴������δ�ɶԵĵ�����Ϊ2���ʴ�Ϊ:1s22s22p63s23p63d84s2��2��
��2���ٸ��ݼ۲���ӶԻ�������,SO42�����������Ӷ�������4���µ��Ӷ���Ϊ(6+2-2��4)��2=0���������ӵ����幹�������������Ρ��ʴ�Ϊ:�������壻
�ڸ�����λ�����ص㣬��[Ni(NH3)6]2+��Ni2+��NH3֮���γɵĻ�ѧ����Ϊ��λ�����ṩ�µ��ӶԵijɼ�ԭ����N���ʴ�Ϊ:��λ����N ��
�۷��Ӽ������������Ӽ�������ǿ�������ķе����좣�PH3�������ݼ۲���ӶԻ������ۣ�������ԭ��N���������Ӷ�������3���µ��Ӷ���Ϊ(5-3)��2=1��������ԭ����sp3�ӻ������ӳ������Σ�����������IJ��ص������Ǽ��Է��ӡ�
�ʴ�Ϊ:���ڣ�NH3���Ӽ���γ���������ԣ�
��3��ͭ�������ڽ���������ͭ���������ɽ������γɵľ��壻ͭʧȥ����ȫ������3d10���ӣ���ʧȥ����4s1���ӣ�����ICu>INi���ʴ�Ϊ:������ͭʧȥ����ȫ������3d10���ӣ���ʧȥ����4s1���ӣ�
��4���ٸ��ݾ�̯�����㣬������ͭԭ�Ӹ���Ϊ6��![]() =3����ԭ�ӵĸ���Ϊ8��
=3����ԭ�ӵĸ���Ϊ8��![]() =1����ͭ����ԭ�ӵ�������Ϊ3��1���ʴ�Ϊ:3��1��
=1����ͭ����ԭ�ӵ�������Ϊ3��1���ʴ�Ϊ:3��1��
�ڸ��������������þ��������ΪCu3Ni�����Ͻ���ܶ�Ϊdg��cm-3��������=m��V��������a=![]() ��107nm���ʴ�Ϊ:
��107nm���ʴ�Ϊ:![]() ��107��
��107��

����Ŀ���ߴ���������Ϊ�ϳ���������Ԫ�������ϵ�ԭ�ϣ���ҵ�Ͽ�����Ȼ�������̷������̿���Fe��Al��Mg��Zn��Ni��Si��Ԫ�أ��Ʊ�����������ͼ��ʾ���ش��������⣺
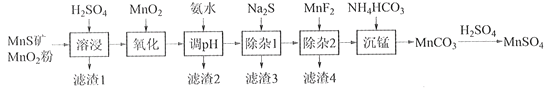
��ؽ�������[c0(Mn+)=0.1 mol��L1]�γ��������������pH��Χ���£�
�������� | Mn2+ | Fe2+ | Fe3+ | Al3+ | Mg2+ | Zn2+ | Ni2+ |
��ʼ������pH | 8.1 | 6.3 | 1.5 | 3.4 | 8.9 | 6.2 | 6.9 |
������ȫ��pH | 10.1 | 8.3 | 2.8 | 4.7 | 10.9 | 8.2 | 8.9 |
��1��������1������S��__________________________��д�����ܽ����ж������������̷�Ӧ�Ļ�ѧ����ʽ____________________________________________________��
��2����������������������MnO2�������ǽ�________________________��
��3������pH��������������Һ��pH��ΧӦ����Ϊ_______~6֮�䡣
��4��������1����Ŀ���dz�ȥZn2+��Ni2+��������3������Ҫ�ɷ���______________��
��5��������2����Ŀ��������MgF2������ȥMg2+������Һ��ȹ��ߣ�Mg2+��������ȫ��ԭ����_____________________________________________________________________��
��6��д���������������ӷ���ʽ___________________________________________________��
��7����״��������Ԫ���Ͽ���Ϊ����ӵ���������ϣ��仯ѧʽΪLiNixCoyMnz2������Ni��Co��Mn�Ļ��ϼ۷ֱ�Ϊ+2��+3��+4����x=y=![]() ʱ��z=___________��
ʱ��z=___________��