题目内容
【题目】(1)将pH=l的盐酸平均分成2份,l份加适量水,另1份加入与该盐酸物质的量浓度相同的适量NaOH溶液后,pH都升高了1,则加入的水与NaOH溶液的体积比为_____。
(2)在25℃下,将pH=3的硫酸溶液和pH=10的NaOH溶液混合,若要使混合后溶液的pH=7,则硫酸溶液和NaOH溶液的体积比约为___
(3)在250C时,有pH为a的盐酸和pH为b的NaOH溶液,取Va L该盐酸,同该NaOH溶液中和,需Vb LNaOH溶液。填空:
①若a + b = 14,则Va∶Vb =_____(填数字)。
②若a + b = 13,则Va∶Vb =_______(填数字)。
③若a + b >14,则Va∶Vb =______(填表达式)。
【答案】11:1 1:10 1 0.1 10a+b-14
【解析】
(1)加水稀释、加入氢氧化钠,氢离子浓度都由10-1变为10-2;
(2)将pH=3的硫酸溶液和pH=10的NaOH溶液混合,若要使混合后溶液的pH=7,酸碱恰好完全反应;
(3)在250C时,Va L pH为a的盐酸和 Vb L pH为b的NaOH溶液完全中和,[H+]=[OH-] ,即![]() 。
。
(1)设每一份盐酸的体积都是1L,氢离子浓度都由10-1变为10-2,设加水的体积是V1,则![]() ,V1=9L;设加氢氧化钠的体积是V2,
,V1=9L;设加氢氧化钠的体积是V2,![]() , V2=
, V2=![]() L,则加入的水与NaOH溶液的体积比为11:1;
L,则加入的水与NaOH溶液的体积比为11:1;
(2)混合后溶液的pH=7,酸碱恰好完全反应,设将xL pH=3的硫酸溶液和yL pH=10的NaOH溶液混合,混合后溶液的pH=7, ![]() ,
,![]() ;
;
(3)①![]() ,
,![]() ,若a + b = 14,则Va∶Vb =1:1;
,若a + b = 14,则Va∶Vb =1:1;
②![]() ,
,![]() ,若a + b = 13,则Va∶Vb =1:10;
,若a + b = 13,则Va∶Vb =1:10;
③![]() ,
,![]() ,若a + b >14,则Va∶Vb = 10a+b-14。
,若a + b >14,则Va∶Vb = 10a+b-14。

 金钥匙试卷系列答案
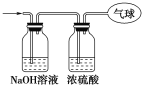
金钥匙试卷系列答案【题目】过氧化钠保存不当容易变质。某课外活动小组为了粗略测定过氧化钠的质量分数,他们称取2.0g样品,并设计用下图装置来测定过氧化钠的质量分数。图中的E和F构成量气装置,用来测定O2的体积。
| |||||
A | B | C | D | E | F |
(1)写出装置A中两种主要玻璃仪器的名称__________、_______。
(2)写出装置A中发生的离子方程式____________________________。
(3)装置B的作用是______________________________。
(4)写出装置C中发生反应的主要化学方程式:______________________________。
(5)装置D中 NaOH的作用是________________________________________。
(6)他们在读出量筒内水的体积数后,折算成标准状况下氧气的体积为224mL,则样品中过氧化钠的质量分数为__________
【题目】硫酸镍(NiSO4)是一种重要的化工原料,广泛应用于电镀、医药、印染等工业。以含镍废催化剂(主要含Ni及少量的Al、Al2O3、Fe和其它不溶于酸、碱的杂质)为原料生产NiSO4·7H2O晶体的两种工艺流程如下:
流程一:
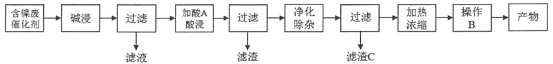
流程二:

已知:部分金属氢氧化物的Ksp近似值如下表所示:
化学式 | Fe(OH)2 | Fe(OH)3 | Al(OH)3 | Ni(OH)2 |
Ksp近似值 | 10-17 | 10-39 | 10-34 | 10-15 |
回答下列问题:
(1)流程一中所用酸A和流程二中所用酸X___________(填“相同”或“不相同”);流程一中滤渣C和流程二中滤渣II___________(填“相同”或“不相同”)。流程二中滤渣I是___________。
(2)流程一中“碱浸”时发生反应的离子方程式为_________________、___________________。
(3)流程二中“净化除杂”包含了两步操作过程:第一步,加入氧化剂H2O2,发生反应的离子方程式是____________________________________________;第二步,调节溶液的pH。
(4)分析比较流程一中操作B和流程二中操作Y后,回答操作Y是___________、___________、过滤、洗涤、干燥,即得产物。
(5)由己知信息列式计算:常温下,Ni2+完全沉淀时的pH值___________。