��Ŀ����
����Ŀ��ijͬѧ����ͼ��ʾװ����ȡ��ˮ���������ʵ�顣
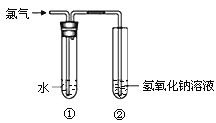
��1��װ�â��з�Ӧ�Ļ�ѧ����ʽ�� ��
��2��ȡ����װ�â��е���Һ������ɫʯ����ֽ�ϣ���ֽ�ȱ�����ɫ��˵����ˮ���е������� ��
��3��ʵ���ҳ���NaOH��Һ���ն���������������Լ�Ҳ������������������ (����ĸ)��
a������NaCl��Һ b������ˮ c��Na2SO3��Һ
��4������֤Cl2��������ǿ��I2��ȡ����װ�â��е���Һ����ʢ��KI��Һ���Թ��У�����������������Ȼ�̼�������ã��۲쵽�������� ��
���𰸡���1��Cl2+2NaOH=NaCl+NaClO+H2O��
��2�����Ժ�Ư���ԣ���3����c��
��4��Һ��ֲ����²�Һ���Ϻ�ɫ���ϲ�Һ����ɫ
��������
�����������1������������������Һ��Ӧ�Ļ�ѧ����ʽ��Cl2+2NaOH=NaCl+NaClO+H2O��
��2��ȡ����װ�â��е���Һ������ɫʯ����ֽ�ϣ���ֽ�ȱ�����ɫ��˵����ˮ���е�������������Ư���ԣ�
��3��������ˮ��Һ�е��ܽ��Ժ�С������������ʳ��ˮ������ˮ���������������ܰ�������������Ϊ�����ƣ���˿���������������Һ������������ѡc��
��4�������͵⻯�ط�Ӧ�����Ȼ��غ͵⣬���������л��ܼ����Ȼ�̼�У��������Ȼ�̼���ܶȴ���ˮ�����۲쵽��������Һ��ֲ����²�Һ���Ϻ�ɫ���ϲ�Һ����ɫ��

 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�