��Ŀ����
X��Y��Z��W��Ϊ10���ӵķ��ӻ����ӡ�X��5��ԭ�Ӻˡ�ͨ��״���£�WΪ��ɫҺ�塣����֮��ת����ϵ��ͼ��ʾ����ش�
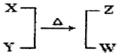
��1����ҵ��ÿ��ȡ1molZҪ�ų�46.2 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��2����ҵ��ȡZ�Ļ�ѧƽ�ⳣ��K��T�Ĺ�ϵ���±���
������������⣺
���ԱȽ�K1��K2�Ĵ�С��K1 K2����д��>����=����<����
�ں��¹̶�����������У����и�������Ϊ�жϸ÷�Ӧ�ﵽ��ѧƽ��״̬�����ݵ��� ���������ĸ����
A�������ڸ����ʵ�Ũ��֮��Ϊ��ѧ��������
B����������ܶȱ��ֲ���
C��������ѹǿ���ֲ���
D�����������Է����������ֲ���
��3��ij��ѧС��ͬѧģ�ҵ������ȡHNO3�������ͼ��ʾװ�ã�����aΪһ���ɳ��������������Ƥ��
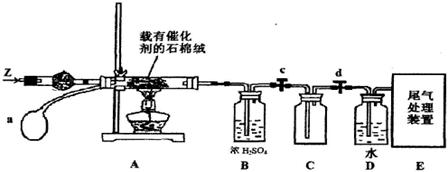
��A�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��B��ŨH2SO4�������� ��
��4��д��Dװ���з�Ӧ�Ļ�ѧ����ʽ ��
��5��a��ͨ����������� ��
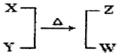
��1����ҵ��ÿ��ȡ1molZҪ�ų�46.2 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��2����ҵ��ȡZ�Ļ�ѧƽ�ⳣ��K��T�Ĺ�ϵ���±���
| T/K | 298 | 398 | 498 | ���� |
| K/(mol��L��1)��2 | 4.1��106 | K1 | K2 | ���� |
������������⣺
���ԱȽ�K1��K2�Ĵ�С��K1 K2����д��>����=����<����
�ں��¹̶�����������У����и�������Ϊ�жϸ÷�Ӧ�ﵽ��ѧƽ��״̬�����ݵ��� ���������ĸ����
A�������ڸ����ʵ�Ũ��֮��Ϊ��ѧ��������
B����������ܶȱ��ֲ���
C��������ѹǿ���ֲ���
D�����������Է����������ֲ���
��3��ij��ѧС��ͬѧģ�ҵ������ȡHNO3�������ͼ��ʾװ�ã�����aΪһ���ɳ��������������Ƥ��

��A�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��B��ŨH2SO4�������� ��
��4��д��Dװ���з�Ӧ�Ļ�ѧ����ʽ ��
��5��a��ͨ����������� ��
��1��N2(g)+3H2 2NH3(g) ��H=��92.4KJ/mol��3�֣�
2NH3(g) ��H=��92.4KJ/mol��3�֣�
(2) �� > ��CD
��3����4NH+5O2 4NO+6H2O ������ˮ�Ͷ���İ���
4NO+6H2O ������ˮ�Ͷ���İ���
(4)3 NO2+H2O=2HNO3+NO��4NO2+O2+2H2O=4HNO3
(5)����NH3��NO
 2NH3(g) ��H=��92.4KJ/mol��3�֣�
2NH3(g) ��H=��92.4KJ/mol��3�֣�(2) �� > ��CD
��3����4NH+5O2
 4NO+6H2O ������ˮ�Ͷ���İ���
4NO+6H2O ������ˮ�Ͷ���İ���(4)3 NO2+H2O=2HNO3+NO��4NO2+O2+2H2O=4HNO3
(5)����NH3��NO
�����������1��5��ԭ�Ӻ˵�10���ӵ���һ��ΪCH4��NH4+NH4+��WΪ��ɫҺ��,10���ӵ���ɫҺ��ΪH2O�����Բ���÷�ӦΪNH4+��OH-��ȡNH3�ķ�Ӧ��XΪNH4+��YΪOH-��ZΪNH3����ҵ��ȡ�����Ļ�ѧ��ӦΪN2+3H2
 2NH3��ÿ��ȡ1molNH3Ҫ�ų�46.2kJ�����������Ը÷�Ӧ���Ȼ�ѧ����ʽΪN2(g)+3H2
2NH3��ÿ��ȡ1molNH3Ҫ�ų�46.2kJ�����������Ը÷�Ӧ���Ȼ�ѧ����ʽΪN2(g)+3H2 2NH3(g) ��H=��92.4KJ/mol
2NH3(g) ��H=��92.4KJ/mol��2������ȡ�����ķ�ӦΪ���ȷ�Ӧ�������¶�������ƽ�������ƶ���ƽ�ⳣ����С������K1>K2
��A����ƽ��ʱ�����ڸ����ʵ�Ũ��֮�Ȳ�һ��Ϊ��ѧ�������ȣ�����B�����ݵ������У�������ܶ�ʼ�ղ��䣬����C�����ŷ�Ӧ�Ľ��У������ѹǿ��С����ƽ��ʱ���ٱ仯����ȷ��D�����ŷ�Ӧ�Ľ��У���������ʵ�����С���������Է�������������ƽ��ʱ���ٱ仯����ȷ����ѡCD��
��3����A�з�����Ӧ�ǰ����Ĵ��������仯ѧ����ʽ��4NH+5O2
 4NO+6H2O
4NO+6H2O��ŨH2SO4������ˮ�ԣ�Ҳ���ԺͰ�����Ӧ������B��ŨH2SO4������������ˮ�Ͷ���İ���
��4�����ڿ����IJ��Ϲ��룬����������ˮ��������ȫ��Ӧ�������ᣬ��ѧ����ʽΪ4NO2+O2+2H2O=4HNO3��3 NO2+H2O=2HNO3+NO
��5��a��ͨ����������þ��Dz�������NH3��NO

��ϰ��ϵ�д�
 �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
�����Ŀ
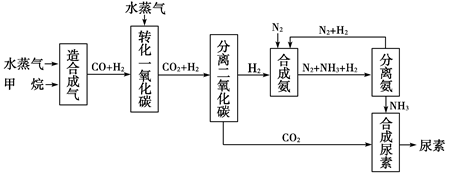
 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��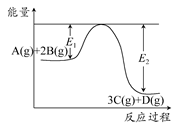
 3C(g)��D(g)����H>0
3C(g)��D(g)����H>0