��Ŀ����
�Ĵ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ��������ͼ��ʾ(ͼ��ijЩת�����輰������δ�г�)��
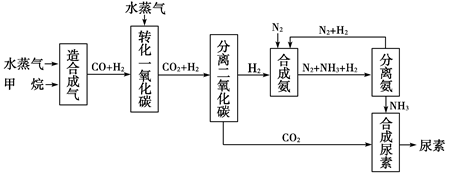
����д���пհף�
(1)��֪0.5 mol������0.5 molˮ������t �桢p kPaʱ����ȫ��Ӧ����һ��
��̼������(�ϳ���)��������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�______________________��
(2)�ںϳɰ���ʵ�����������У�����ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ����������������������ĵ���������ѭ�����ã�ͬʱ���䵪���������������û�ѧ��Ӧ���ʺͻ�ѧƽ��Ĺ۵�˵����ȡ�ô�ʩ�����ɣ�
________________________________________________________________��
(3)������ϳɰ�����ת����Ϊ75%ʱ����5.60��107 L����Ϊԭ���ܹ��ϳ�________L������(����������ڱ�״���²ⶨ)
(4)��֪���صĽṹ��ʽΪ ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
��__________________�� ��_________________��

����д���пհף�
(1)��֪0.5 mol������0.5 molˮ������t �桢p kPaʱ����ȫ��Ӧ����һ��
��̼������(�ϳ���)��������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�______________________��
(2)�ںϳɰ���ʵ�����������У�����ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ����������������������ĵ���������ѭ�����ã�ͬʱ���䵪���������������û�ѧ��Ӧ���ʺͻ�ѧƽ��Ĺ۵�˵����ȡ�ô�ʩ�����ɣ�
________________________________________________________________��
(3)������ϳɰ�����ת����Ϊ75%ʱ����5.60��107 L����Ϊԭ���ܹ��ϳ�________L������(����������ڱ�״���²ⶨ)
(4)��֪���صĽṹ��ʽΪ
 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ����__________________�� ��_________________��
(1)CH4(g)��H2O(g)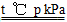 CO(g)��3H2(g) ��H����2a kJ��mol��1
CO(g)��3H2(g) ��H����2a kJ��mol��1
(2)��������������Ũ������������Ӧ���ʣ���С������Ũ�ȡ���������������Ũ�Ⱦ�������ƽ��������Ӧ�����ƶ�
(3)8.4��107
(4)��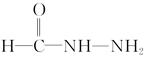 ����NH4N��C��O
����NH4N��C��O
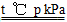 CO(g)��3H2(g) ��H����2a kJ��mol��1
CO(g)��3H2(g) ��H����2a kJ��mol��1(2)��������������Ũ������������Ӧ���ʣ���С������Ũ�ȡ���������������Ũ�Ⱦ�������ƽ��������Ӧ�����ƶ�
(3)8.4��107
(4)��
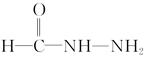 ����NH4N��C��O
����NH4N��C��O(1)0.5 mol CH4��ȫ��Ӧ����a kJ��������1 mol CH4��ȫ��Ӧ����2a kJ�������Ȼ�ѧ����ʽΪCH4(g)��H2O(g)=CO(g)��3H2(g)����H����2a kJ��mol��1��
(2)����ѭ����������������Ӧ��Ũ�ȣ�����Ӧ���ʣ����ת���ʣ�����NH3Ũ��������ƽ�������ƶ���
(3)�ɷ�Ӧ��ѧ����ʽ����ת����ϵ1CH4��3H2��2NH3��V(NH3)��5.60��107 L��75%��2��8.4��107 L��
(4)����д�� �ṹ����������������ӡ���д���칹��
�ṹ����������������ӡ���д���칹��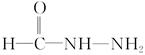 ��NH4N��C��O�Ľṹ��ʽ��
��NH4N��C��O�Ľṹ��ʽ��
(2)����ѭ����������������Ӧ��Ũ�ȣ�����Ӧ���ʣ����ת���ʣ�����NH3Ũ��������ƽ�������ƶ���
(3)�ɷ�Ӧ��ѧ����ʽ����ת����ϵ1CH4��3H2��2NH3��V(NH3)��5.60��107 L��75%��2��8.4��107 L��
(4)�����
 �ṹ����������������ӡ���д���칹��
�ṹ����������������ӡ���д���칹�� ��NH4N��C��O�Ľṹ��ʽ��
��NH4N��C��O�Ľṹ��ʽ��
��ϰ��ϵ�д�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
�����Ŀ
 P4O10����֪�������л�ѧ����Ҫ���յ������ֱ�Ϊ:P��P a kJ��mol-1��P��O b kJ��mol-1��P
P4O10����֪�������л�ѧ����Ҫ���յ������ֱ�Ϊ:P��P a kJ��mol-1��P��O b kJ��mol-1��P O c kJ��mol-1��O
O c kJ��mol-1��O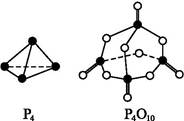
 2PbSO4+2H2O
2PbSO4+2H2O CaO+CO2��
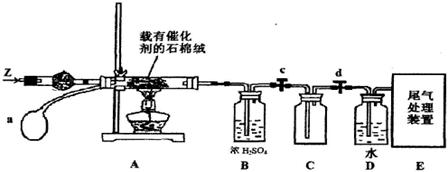
CaO+CO2�� HBr��H�������Է�Ӧ���̵�ʾ��ͼ������������ȷ����(����)
HBr��H�������Է�Ӧ���̵�ʾ��ͼ������������ȷ����(����)
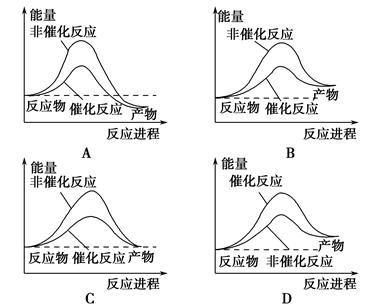



 C(g)��H1=-Q1 kJ/mol
C(g)��H1=-Q1 kJ/mol