��Ŀ����
��5�֣���ú��Ϊȼ�Ͽ�ͨ����������;����
;���� C(s)+O2(g)=====CO2(g)����H1��0 ��
;���� ���Ƴ�ˮú����
C(s)+H2O(g)=====CO(g)+H2(g)����H2��0 ��
��ȼ��ˮú����
2CO(g)+O2(g)=====2CO2(g)����H3��0 ��
2H2(g)+O2(g)=====2H2O(g)����H4��0 ��
��ش��������⣺
(1);����ų�������������_________(����ڡ������ڡ���С�ڡ�);����ų���������
(2)��H1����H2����H3����H4����ѧ��ϵʽ��_______________��
(3)����֪���� C(s)��O2(g)��CO2(g)�� DH����393.5 kJ��mol-1
�� 2CO(g)��O2(g)��2CO2(g)�� DH��-566 kJ��mol-1
�� TiO2(s)��2Cl2(g)��TiCl4(s)��O2(g)�� DH��+141 kJ��mol-1
��TiO2(s)��2Cl2(g)��2C(s)��TiCl4(s)��2CO(g)��DH�� ��
;���� C(s)+O2(g)=====CO2(g)����H1��0 ��
;���� ���Ƴ�ˮú����
C(s)+H2O(g)=====CO(g)+H2(g)����H2��0 ��
��ȼ��ˮú����
2CO(g)+O2(g)=====2CO2(g)����H3��0 ��
2H2(g)+O2(g)=====2H2O(g)����H4��0 ��
��ش��������⣺
(1);����ų�������������_________(����ڡ������ڡ���С�ڡ�);����ų���������
(2)��H1����H2����H3����H4����ѧ��ϵʽ��_______________��
(3)����֪���� C(s)��O2(g)��CO2(g)�� DH����393.5 kJ��mol-1
�� 2CO(g)��O2(g)��2CO2(g)�� DH��-566 kJ��mol-1
�� TiO2(s)��2Cl2(g)��TiCl4(s)��O2(g)�� DH��+141 kJ��mol-1
��TiO2(s)��2Cl2(g)��2C(s)��TiCl4(s)��2CO(g)��DH�� ��
��5�֣���1�����ڣ�1�֣� (2 ) ��H1 = ��H2 + (3) ��80 kJ��mol-1
(3) ��80 kJ��mol-1
 (3) ��80 kJ��mol-1
(3) ��80 kJ��mol-1��1�����������غ㶨�ɿ�֪������;���зų�����������ȵġ�
��2�����ݸ�˹���ɿ�֪���ڣ����ۣ��ܣ���2���õ���Ӧ�٣����Ԧ�H1 = ��H2 + ��
��
��3�����ݸ�˹���ɿ�֪���ۣ��١�2���ڼ��õ�TiO2(s)��2Cl2(g)��2C(s)��TiCl4(s)��2CO(g)������DH����393.5kJ/mol��2��141kJ/mol��566kJ/mol����80kJ/mol.
��2�����ݸ�˹���ɿ�֪���ڣ����ۣ��ܣ���2���õ���Ӧ�٣����Ԧ�H1 = ��H2 +
 ��
����3�����ݸ�˹���ɿ�֪���ۣ��١�2���ڼ��õ�TiO2(s)��2Cl2(g)��2C(s)��TiCl4(s)��2CO(g)������DH����393.5kJ/mol��2��141kJ/mol��566kJ/mol����80kJ/mol.

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 CH3OH(g)+H2O(g) ��H = ��49.0 kJ��mol-1
CH3OH(g)+H2O(g) ��H = ��49.0 kJ��mol-1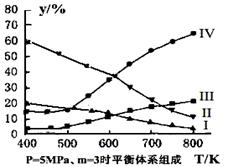

 2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������(S)��ʱ��(t)�ı仯���ߣ��������ı����S1��S2 ������ͼ�л���NO��Ũ����T1��S2 �����´ﵽƽ������еı仯���ߣ���ע��������
2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������(S)��ʱ��(t)�ı仯���ߣ��������ı����S1��S2 ������ͼ�л���NO��Ũ����T1��S2 �����´ﵽƽ������еı仯���ߣ���ע�������� H2(g)��CO(g)�� ��H����131.3 kJ����S����133.7 J/K
H2(g)��CO(g)�� ��H����131.3 kJ����S����133.7 J/K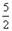 O2=2CO2(g)+H2O(l) ��H��-1300kJ/mol��˵���У���ȷ���ǣ��� ��
O2=2CO2(g)+H2O(l) ��H��-1300kJ/mol��˵���У���ȷ���ǣ��� ��