��Ŀ����
(11��)��CO2Ϊ̼Դ��ȡ��̼�л����Ϊ�����о����㣬����ΪCO2������ȡ��̼��������ѧ���ݣ�
��ӦI�� CO2(g)+3H2(g) CH3OH(g)+H2O(g) ��H = ��49.0 kJ��mol-1
CH3OH(g)+H2O(g) ��H = ��49.0 kJ��mol-1
��ӦII��2CO2(g)+6H2(g) CH3CH2OH(g)+3H2O(g) ��H =" ��173.6" kJ��mol-1
CH3CH2OH(g)+3H2O(g) ��H =" ��173.6" kJ��mol-1
(1)д����CH3CH2OH+_____ 2CH3OH���Ȼ�ѧ����ʽΪ��__________________��
2CH3OH���Ȼ�ѧ����ʽΪ��__________________��
(2)��һ�������£����ڷ�ӦI��������㶨���ܱ������У��ﵽƽ��ı�־��__ (����ĸ)
a��CO2��CH3OH Ũ����ȡ���������b��H2O�İٷֺ������ֲ���
c��H2��ƽ����Ӧ����Ϊ0�������� d��v��(CO2)=3v��(H2)��
e.���������ܶȲ��ٷ����ı�
f. ��������ƽ����Է����������ٷ����ı�
���ƽ�ⳣ��Kֵ��÷�Ӧ������������������(����ĸ)
a��һ��������Ӧ�����ƶ������� b����ƽ���ƶ�ʱ����Ӧ������������С
c��һ�����淴Ӧ�����ƶ������� d����ƽ���ƶ�ʱ�淴Ӧ�����ȼ�С������
���������㶨����������CO2�ķ�Ӧ���ʣ����Բ�ȡ�ķ�Ӧ������ (����ĸ) ��
�ﵽƽ��������H2ת���ʵ���_______________(����ĸ)
a�������¶� b������H2 c����ȥ�״� d���������
��3�����ܱ������У����ڷ�ӦII�У��о�Ա�������Ҵ�Ϊ�о�������5MPa��m= n(H2)/n(CO2)=3ʱ����ò�ͬ�¶���ƽ����ϵ�и������ʵ����������y%����ͼ��ʾ�����ʾCH3CH2OH����������ߵ��� ����ʾCO2������������ߵ��� ��


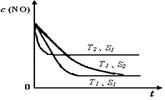
��4��������һ��ʱ�������������ı���������ѧ��Ӧ���ʡ���ͼ�Ƿ�Ӧ��2NO(g) + 2CO(g) 2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������(S)��ʱ��(t)�ı仯���ߣ��������ı����S1��S2 ������ͼ�л���NO��Ũ����T1��S2 �����´ﵽƽ������еı仯���ߣ���ע��������
2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������(S)��ʱ��(t)�ı仯���ߣ��������ı����S1��S2 ������ͼ�л���NO��Ũ����T1��S2 �����´ﵽƽ������еı仯���ߣ���ע��������
��ӦI�� CO2(g)+3H2(g)
 CH3OH(g)+H2O(g) ��H = ��49.0 kJ��mol-1
CH3OH(g)+H2O(g) ��H = ��49.0 kJ��mol-1��ӦII��2CO2(g)+6H2(g)
 CH3CH2OH(g)+3H2O(g) ��H =" ��173.6" kJ��mol-1
CH3CH2OH(g)+3H2O(g) ��H =" ��173.6" kJ��mol-1(1)���CH3CH2OH+_____
 2CH3OH���Ȼ�ѧ����ʽΪ��__________________��
2CH3OH���Ȼ�ѧ����ʽΪ��__________________��(2)��һ�������£����ڷ�ӦI��������㶨���ܱ������У��ﵽƽ��ı�־��__ (����ĸ)
a��CO2��CH3OH Ũ����ȡ���������b��H2O�İٷֺ������ֲ���
c��H2��ƽ����Ӧ����Ϊ0�������� d��v��(CO2)=3v��(H2)��
e.���������ܶȲ��ٷ����ı�
f. ��������ƽ����Է����������ٷ����ı�
���ƽ�ⳣ��Kֵ��÷�Ӧ������������������(����ĸ)
a��һ��������Ӧ�����ƶ������� b����ƽ���ƶ�ʱ����Ӧ������������С
c��һ�����淴Ӧ�����ƶ������� d����ƽ���ƶ�ʱ�淴Ӧ�����ȼ�С������
���������㶨����������CO2�ķ�Ӧ���ʣ����Բ�ȡ�ķ�Ӧ������ (����ĸ) ��
�ﵽƽ��������H2ת���ʵ���_______________(����ĸ)
a�������¶� b������H2 c����ȥ�״� d���������
��3�����ܱ������У����ڷ�ӦII�У��о�Ա�������Ҵ�Ϊ�о�������5MPa��m= n(H2)/n(CO2)=3ʱ����ò�ͬ�¶���ƽ����ϵ�и������ʵ����������y%����ͼ��ʾ�����ʾCH3CH2OH����������ߵ��� ����ʾCO2������������ߵ��� ��


��4��������һ��ʱ�������������ı���������ѧ��Ӧ���ʡ���ͼ�Ƿ�Ӧ��2NO(g) + 2CO(g)
 2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������(S)��ʱ��(t)�ı仯���ߣ��������ı����S1��S2 ������ͼ�л���NO��Ũ����T1��S2 �����´ﵽƽ������еı仯���ߣ���ע��������
2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������(S)��ʱ��(t)�ı仯���ߣ��������ı����S1��S2 ������ͼ�л���NO��Ũ����T1��S2 �����´ﵽƽ������еı仯���ߣ���ע����������1��CH3CH2OH(g)+H2O(g)= 2CH3OH(g) H = +75.6 kJ��mol-1
��2��bcf ��ad ,bd ac
��3�� I III (2��)
��4��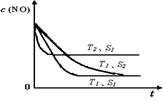
��2��bcf ��ad ,bd ac
��3�� I III (2��)
��4��

�����������1��I��2-II��CH3CH2OH(g)+H2O(g)= 2CH3OH(g) ��H = +75.6 kJ��mol-1
��2�������÷�Ӧ��ƽ��ʱ����Ũ��֮��Ĺ�ϵ���жϴ�ƽ�⣬a�����淴Ӧ��ƽ��ʱΪ��̬ƽ�⣬���ʲ�Ϊ0��c����CO2��H2�ļ���ϵ��֮��Ϊ1��3������v��(CO2)=3v��(H2)���淴Ӧ���ʲ��ȣ�d��������bef�����жϴ�ƽ��ı�־��I��ӦΪ���ȷ�Ӧ��K����˵�����£�ƽ�������ƶ������ѡad�������CO2�ķ�Ӧ���ʣ����������¶ȣ�����ѹǿ������Ũ�ȣ�ʹ�ô��������ѡbd�����H2ת���ʣ�ƽ��Ҫ�����ƶ������ѡac��
��3����ӦIIΪ���ȷ�Ӧ�������¶����ߣ��Ҵ����������Ӧ�ü�С�����ݷ�Ӧ��֪CO2��H2��ʹ��ȫת�����Ҵ�������������Ҳֻ��25%����������I�DZ�ʾ�Ҵ���������������¶����ߣ�CO2���������Ӧ������CO2��������������ܳ���25%����������III�DZ�ʾCO2���������
��4�����������С����Ч�������ʼ��������Ǵ������ܸı�ƽ�⣬����T1���¶��´�ƽ�⣬NO��Ũ�Ȳ��䣬���Ǵ�ƽ���ʱ�����ӣ����Ա仯������ͼ��

��ϰ��ϵ�д�
�����Ŀ
 2HCl(g)����H����184.6kJ��mol��1��
2HCl(g)����H����184.6kJ��mol��1��





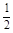 O2(g) ��H2O(l) ��H2���C285.8 kJ/mol
O2(g) ��H2O(l) ��H2���C285.8 kJ/mol