��Ŀ����
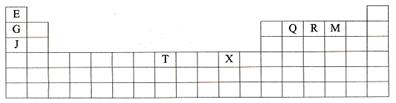
��15�֣���֪��A��B��C��D��E��F����Ԫ�غ˵�����������������ڱ���ǰ�����ڵ�Ԫ�ء�����Aԭ�Ӻ���������δ�ɶԵ��ӣ�B�ڵ��������һ�����B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵ��۵���ͬ����Ԫ���γɵĵ���������ߵģ�Fԭ�Ӻ���������������B��ͬ��λ�ڵ������ڵ�IB�壬�����������Ϣ���ش��������⣺
��1��A��B��C��D�ĵ�һ��������С�����˳��Ϊ ��(��Ԫ�ط��ű�ʾ)
��2��B���Ȼ�����۵��D���Ȼ�����۵� �����ͣ��������� ��
��3��E�������������ӵĿռ乹���� ��
��4��F�ĺ�������Ų�ʽ�� ��F�ĸ�������A�ļ��⻯���γɵ������ӵĻ�ѧʽΪ .
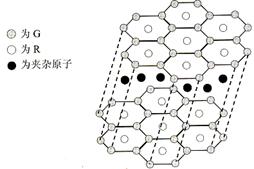
��5��A��F�γ�ij�ֻ�����ľ����ṹ��ͼ��ʾ�����仯ѧʽΪ ������ɫ���ʾFԭ�ӣ�
��6��A��C�γɵĻ�������и߷е��Ӳ�ȣ���һ���������ǽ������ϣ��侧���������Ļ�ѧ������Ϊ ��
��1��A��B��C��D�ĵ�һ��������С�����˳��Ϊ ��(��Ԫ�ط��ű�ʾ)
��2��B���Ȼ�����۵��D���Ȼ�����۵� �����ͣ��������� ��
��3��E�������������ӵĿռ乹���� ��
��4��F�ĺ�������Ų�ʽ�� ��F�ĸ�������A�ļ��⻯���γɵ������ӵĻ�ѧʽΪ .
��5��A��F�γ�ij�ֻ�����ľ����ṹ��ͼ��ʾ�����仯ѧʽΪ ������ɫ���ʾFԭ�ӣ�

��6��A��C�γɵĻ�������и߷е��Ӳ�ȣ���һ���������ǽ������ϣ��侧���������Ļ�ѧ������Ϊ ��
��1��Na��Al��Si��N��2�֣�
��2���ߣ�1�֣�NaClΪ���Ӿ����SiCl4Ϊ���Ӿ��壨2�֣�
��3��ƽ���������Σ�2�֣�
��4��1s22s22p63s23p63d104s1���� 3d104s1����2�֣� 2+��2�֣�
��5��Cu3 N ��2�֣� ��6�����ۼ���2�֣�
��2���ߣ�1�֣�NaClΪ���Ӿ����SiCl4Ϊ���Ӿ��壨2�֣�
��3��ƽ���������Σ�2�֣�
��4��1s22s22p63s23p63d104s1���� 3d104s1����2�֣� 2+��2�֣�
��5��Cu3 N ��2�֣� ��6�����ۼ���2�֣�
����Ԫ�صĽṹ���й����ʿ�֪N��Na��Al��Si��S��Cu��
��1���ǽ�����ѹǿ����һ������Խ������A��B��C��D�ĵ�һ��������С�����˳��ΪNa��Al��Si��N��
��2������NaClΪ���Ӿ����SiCl4Ϊ���Ӿ��壬�����Ȼ��Ƶ��۵����SiCl4�ġ�
��3��������������Sԭ�ӵŶԵ��Ӷ����ǣ�6��2��3����2��0����������������ƽ�������νṹ��
��4�����ݹ���ԭ����֪��Cuԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s1���� 3d104s1����ͭ���ӺͰ����γɵ�����λ�������а��������壬��ѧʽΪ[Cu(NH)3]42����
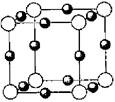
(5)���ݾ����ṹ��֪����ԭ������8��1/8��1��ͭԭ������12��1/4=3�����Ի�ѧʽΪCu3 N��
��6��A��C�γɵĻ�������и߷е��Ӳ�ȣ���һ���������ǽ������ϣ���˵���þ�����ԭ�Ӿ��壬���еĻ�ѧ���ǹ��ۼ���
��1���ǽ�����ѹǿ����һ������Խ������A��B��C��D�ĵ�һ��������С�����˳��ΪNa��Al��Si��N��
��2������NaClΪ���Ӿ����SiCl4Ϊ���Ӿ��壬�����Ȼ��Ƶ��۵����SiCl4�ġ�
��3��������������Sԭ�ӵŶԵ��Ӷ����ǣ�6��2��3����2��0����������������ƽ�������νṹ��
��4�����ݹ���ԭ����֪��Cuԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s1���� 3d104s1����ͭ���ӺͰ����γɵ�����λ�������а��������壬��ѧʽΪ[Cu(NH)3]42����
(5)���ݾ����ṹ��֪����ԭ������8��1/8��1��ͭԭ������12��1/4=3�����Ի�ѧʽΪCu3 N��
��6��A��C�γɵĻ�������и߷е��Ӳ�ȣ���һ���������ǽ������ϣ���˵���þ�����ԭ�Ӿ��壬���еĻ�ѧ���ǹ��ۼ���

��ϰ��ϵ�д�
�����Ŀ




 ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���塣B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1��18���еĵ�7��Ԫ�ء�D��ԭ��������EС5��D��B���γ����ӻ�����侧���ṹ����ͼ��
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���塣B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1��18���еĵ�7��Ԫ�ء�D��ԭ��������EС5��D��B���γ����ӻ�����侧���ṹ����ͼ��