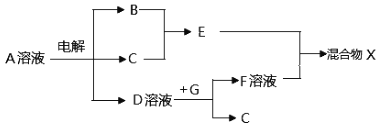
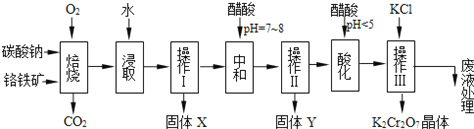
��Ŀ����
����Ŀ������ʮ�Ŵ�������ӿ���̬�������Ƹĸ���������й�������չ�����Դ�Խ��������й�������Ҫ���塣������һ�ָ�Ч��ࡢ���߷�չDZ������Դ��
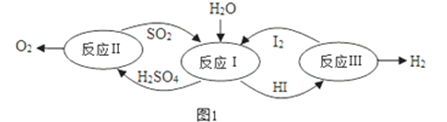
��1����̫����Ϊ��Դ���Ȼ�ѧ���ѭ���ֽ�ˮ��һ�ָ�Ч������Ⱦ�����ⷽ�����䷴Ӧ������ͼ1��ʾ��
�ٷ�Ӧ������ӷ���ʽ��______________________________________________����Ӧ��õ��IJ�����I2���з��룬�ò������Һ�ڹ���I2�Ĵ����»�ֳ����㣺����Ũ��I2��H2SO4��ͺ���Ũ��I2��HI�㡣������Ũ�ȼ�⣬H2SO4��Һ����c��H+����c��SO42-��=2.06��1�����ֵ����2��ԭ���� _______________________ ��
�ڷ�Ӧ��2H2SO4��l��=2SO2��g��+O2��g��+ 2H2O��g����H = +550kJmo1-1������������Ӧ��ɣ�����H2SO4��l��=SO3��g��+ H2O��g����H = +177kJmo1-1������SO3��g���ֽ⣬д��SO3��g���ֽ���Ȼ�ѧ����ʽ ______________________________��
��2����ҵ�����÷�ӦC��s��+2H2O��g��![]() CO2��g��+2H2��g�� ��H��0 Ҳ���Ʊ�������һ�������£���C��s����H2O��g���ֱ����ס��������ܱ�����������Ӧ����������������ʾ��
CO2��g��+2H2��g�� ��H��0 Ҳ���Ʊ�������һ�������£���C��s����H2O��g���ֱ����ס��������ܱ�����������Ӧ����������������ʾ��
���� | �ݻ�/L | �¶�/�� | ��ʼ��/mol | ƽ����/mol | |
C��s�� | H2O��g�� | H2��g�� | |||
�� | 2 | T1 | 2 | 4 | 3.2 |
�� | 1 | T2 | 1 | 2 | 1.2 |
��T1��ʱ���÷�Ӧ��ƽ�ⳣ��K= ______ ��T1 ______ T2�����������=����������
�����������дﵽƽ������ʱ��Ϊ3min����Ӧ���е�1.5minʱ��H2O��g�������ʵ���Ũ�� ______����ѡ����ĸ����
A��=1.4mol/L B����1.4mol/L C����1.4mol/L
��3����ҵ�ϻ��ɲ��õ绯ѧ������H2S������ȡ�������÷�������̵�ʾ��ͼ��ͼ��ʾ��
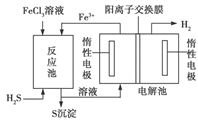
�ٷ�Ӧ���з�Ӧ��������������Һ������ʽ����Ŀ����____________________��
�ڷ�Ӧ���з�����Ӧ�����Һ������أ�����ܷ�Ӧ�����ӷ���ʽΪ_____________��
���𰸡� SO2+2H2O+I2=SO42-+2I-+4H+ ������к�������HI����HI����������� 2SO3��g��![]() 2SO2��g��+O2��g����H = +196kJmol-1 12.8 �� B ��������Һ������ʽ��Ŀ��������Ӧ��Ӵ������ʹ��Ӧ����� 2Fe2����2H��
2SO2��g��+O2��g����H = +196kJmol-1 12.8 �� B ��������Һ������ʽ��Ŀ��������Ӧ��Ӵ������ʹ��Ӧ����� 2Fe2����2H��![]() 2Fe3����H2��
2Fe3����H2��
����������1���ٷ�Ӧ���ж�������͵�ˮ��Ӧ�������������ᣬ�÷�Ӧ�ĵ����ӷ���ʽ��SO2+2H2O+I2=SO42-+2I-+4H+����Ӧ��õ��IJ�����I2���з��룬�ò������Һ�ڹ���I2�Ĵ����»�ֳ����㣺����Ũ��I2��H2SO4��ͺ���Ũ��I2��HI�㡣������Ũ�ȼ�⣬H2SO4��Һ����c��H+����c��SO42-��=2.06��1�����ֵ����2��ԭ������������к�������HI����HI�������������
�ڷ�Ӧ����2H2SO4��l��= 2SO2��g��+O2��g��+ 2H2O��g����H = +550kJmo1-1������������Ӧ��ɣ�����H2SO4��l��= SO3��g��+ H2O��g����H = +177kJmo1-1������SO3��g���ֽ⡣���ݸ�˹���ɣ�����II-��![]() ���ɵ�SO3��g���ֽ���Ȼ�ѧ����ʽ2SO3��g��
���ɵ�SO3��g���ֽ���Ȼ�ѧ����ʽ2SO3��g��![]() 2SO2��g��+O2��g����H = +196kJmol-1 ��
2SO2��g��+O2��g����H = +196kJmol-1 ��
��2����T1��ʱ���������У������ı仯��Ϊ3.2mol����ˮ�Ͷ�����̼�ı仯��Ϊ3.2mol��1.6mol��H2O��g���� CO2��g����H2��g����ƽ�����ֱ�Ϊ0.8mol��1.6mol��3.2mol��H2O��g���� CO2��g����H2��g����ƽ��Ũ�ȷֱ�Ϊ0.4mol/L��0.8mol/L��1.6mol/L���÷�Ӧ��ƽ�ⳣ��K=![]() ��T2��ʱ���������У�H2O��g���� CO2��g����H2��g����ƽ��Ũ�ȷֱ�Ϊ0.8mol/L��0.6mol/L��1.2mol/L��ƽ�ⳣ��K=
��T2��ʱ���������У�H2O��g���� CO2��g����H2��g����ƽ��Ũ�ȷֱ�Ϊ0.8mol/L��0.6mol/L��1.2mol/L��ƽ�ⳣ��K=![]() �����ڸ÷�ӦΪ���ȷ�Ӧ���¶�Խ��ƽ�ⳣ��Խ������T1 ��T2��
�����ڸ÷�ӦΪ���ȷ�Ӧ���¶�Խ��ƽ�ⳣ��Խ������T1 ��T2��
�����������дﵽƽ������ʱ��Ϊ3min��ƽ��ʱ��H2O��g���ı仯������1.2mol����ΪŨ��Խ��ѧ��Ӧ����Խ�죬���Ե���Ӧ���е�1.5minʱ��H2O��g���ı仯������0.6mol�����Դ�ʱH2O��g�������ʵ���Ũ��С��1.4mol/L��ѡB��
��3���ٷ�Ӧ���з�Ӧ��������������Һ������ʽ����Ŀ���Dz�������Һ������ʽ��Ŀ��������Ӧ��Ӵ������ʹ��Ӧ�������
����ͼ����Ϣ��֪����Ӧ���з���2Fe3����H2S=S��+2Fe2����2H������Ӧ�����Һ������أ�������Fe2��������ΪFe3����������H������ԭΪH2������ܷ�Ӧ�����ӷ���ʽΪ2Fe2����2H��![]() 2Fe3����H2����
2Fe3����H2����

 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д�