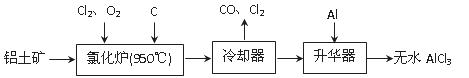
��Ŀ����
п�̵�أ��׳Ƹɵ�أ��������е������ܴ�����п�̵�صĹ���ͼ��ͼ��a����ʾ��
�ش��������⣺
��1����ͨп�̵�طŵ�ʱ��������Ҫ��ӦΪ��
Zn+2NH4Cl+2MnO2=Zn(NH3)2Cl2+2MnOOH
�ٸõ���У�����������Ҫ��________������ʵ���Ҫ�ɷ���______��������������Ҫ��Ӧ��________��
������ͨп�̵����ȣ�����п�̵�ص��ŵ㼰��������_______��
��2��ͼ��b����ʾ�������÷Ͼ���ͨп�̵�ص�һ�ֹ��գ������ǷϾɵ����ʵ�ʴ��ڵ�����������������
��ͼ��b���в���Ļ�ѧʽ�ֱ�ΪA_______��B________��
�ڲ���a�еõ��ۿ����Ҫ�ɷ���K2MnO4������b�У���ɫ��K2MnO4��Һ��Ӧ��������ɫ��Һ��һ�ֺں�ɫ���壬�÷�Ӧ�����ӷ���ʽΪ_______��
�۲��ö��Ե缫���K2MnO4��ҺҲ�ܵõ�������D�����������õ�����Ҫ������ �����ѧʽ��
��1����Zn NH4Cl MnO2+NH4++e-=MnOOH+NH3
�ڼ��Ե�ز���������ʵ�й¶����Ϊ���ĵĸ�����װ�ڵ�ص��ڲ������Ե�ص�ʹ�������ϳ�����Ϊ���������ڼ��Ե�����б������Ե�����е��ȶ�����ߡ�
��2����ZnCl2��NH4Cl��3MnO42-+2CO2=2MnO4-+MnO2��+2CO32-��H2
����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д��������ӷ���ʽ������ȷ����
| A������������ˮ�Ʊ������Cl2��H2O��2H����Cl����ClO�� |
| B����Fe(OH)2�м���������ϡHNO3��Fe(OH)2��2H����Fe2����2H2O |
| C����̼�������Һ�м�������������������Һ��NH4+��OH����NH3��H2O |
| D����������Һ�м������������������Һ��Al3����2SO42����2Ba2����4OH����2BaSO4����AlO2-��2H2O |
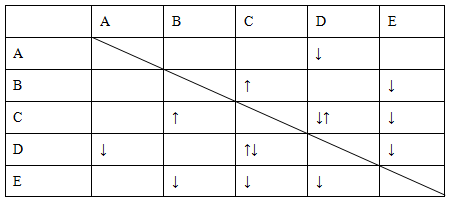
(1)ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
| ������ | 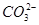 �� ��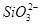 �� �� ��Cl�� ��Cl�� |
| ������ | Al3����Cu2����Mg2���� ��Na�� ��Na�� |
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ���(n)������Լ�Y�����(V)�Ĺ�ϵ��ͼ��ʾ��
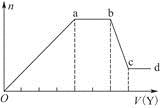
����Y�����ᣬ����Һ�к��еĽ�����������____________________________��
ab�η�����Ӧ�������ӷ���ʽΪ______________________________________��
����Oa����Y��Һ��Ӧ�����ӵ����ʵ���֮��Ϊ__________[Ҫ�������ӷ��ţ���n(Na��)]��
����Y��NaOH��Һ����bc�η�Ӧ�����ӷ���ʽΪ
_________________________________________________________________��
�����������ӵ�ˮ�����أ�����H����OH����Ӱ�죬����Һ��ֻ����4�����ӣ������ǵ����Ӹ�����Ϊ____________________________________________[����������ǰ���������ں���ǰ���ͼ��ں��˳������]��
(2)��Ϊ�ڢ�A��Ԫ�أ����ĵ��ʺͻ�������ijЩ���ʵĻ�ѧ����������������֮������֪��Ԫ�ؾ����������ʣ�
Sn4����Sn=2Sn2����
2Sn2����O2��4H��=2Sn4����2H2O��
2H����SnO
 Sn(OH)2
Sn(OH)2 Sn2����2OH����
Sn2����2OH�����Իش�
�����������ᣬ����Ӧ�����Һ��ͨ���������йط�Ӧ������������Ӧ�仯����д���йط�Ӧ�����ӷ���ʽ��
___________________________________��________________________________��
�ڽ�������Һ���ɺ�����������ù��壬�仯����������FeCl3��Һ��Ӧ�ı仯�������õ��Ĺ���������(����ʽ)__________��
��������SnCl2��Һ������ļ���Һ��Ӧ�ķ�����Sn(OH)2, �ü���__________��
ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
| ������ | CO32����SiO32����AlO2����Cl�� |
| ������ | Al3����Fe3����Mg2����NH4����Na�� |
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ���(n)������Լ����(V)�Ĺ�ϵͼ��ʾ��
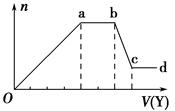
(1)��Y�����ᣬ��Oa��ת��Ϊ����������(ָ��Դ��X��Һ�ģ���ͬ)�� ��
ab�η�����Ӧ�������� ��bc�η�����Ӧ�����ӷ���ʽΪ ��
(2)��Y��NaOH��Һ����X��һ�����е������� ��ab�η�Ӧ�����ӷ���ʽΪ ��
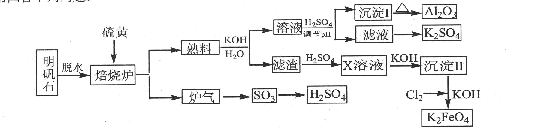
�ú���Al2O3��SiO2������FeO��xFe2O3�������Ʊ�Al2(SO4)3��18H2O��������������(���ֲ�����������)��
��.�������м������ϡH2SO4�����ˣ�
��.����Һ�м������KMnO4��Һ��������Һ��pHԼΪ3��
��.���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ��
��������MnSO4���Ϻ�ɫ��ʧ�����ˣ�
V��Ũ�����ᾧ�����룬�õ���Ʒ��
(1)H2SO4�ܽ�Al2O3�����ӷ���ʽ��_______________________��
(2)��MnO4������Fe2�������ӷ���ʽ����������
1MnO4����Fe2����________===1Mn2����Fe3����________
(3)��֪��
�����������������pH
| | Al(OH)3 | Fe(OH)2 | Fe(OH)3 |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
ע���������ӵ���ʼŨ��Ϊ0.1 mol��L��1
���ݱ������ݽ��Ͳ�����Ŀ�ģ�________________________��
(4)��֪��һ�������£�MnO4������Mn2����Ӧ����MnO2��
�����ij����м���ŨHCl�����ȣ���˵�������д���MnO2��������____________________________��
�ܢ��м���MnSO4��Ŀ����__________________________��
 2Al2O3+6SO2��+3O2����������102gAl2O3��ת�Ƶĵ�����ĿΪ .
2Al2O3+6SO2��+3O2����������102gAl2O3��ת�Ƶĵ�����ĿΪ .