��Ŀ����
����Ŀ����ҵ���������Ҫ��ӦΪ4NH3(g)+5O2(g)4NO(g)+6H2O(l)��H
(1)��֪��������ȼ����Ϊ285.8kJmol-1
N2(g)+3H2(g)2NH3(g)��H=-92.4kJmol-1
N2(g)+O2(g)2NO(g)��H=+180.6kJmol-1
��������ҵ���������Ҫ��Ӧ����H=______��
(2)���ݻ��̶����ܱ������з���������Ӧ�������ڲ������ʵ����ʵ���Ũ�����±���
Ũ�� | c(NH3)(molL-1) | c(O2)(molL-1) | c(NO)(molL-1) |
��ʼ | 0.8 | 1.6 | 0 |
��2min | 0.6 | a | 0.2 |
��4min | 0.3 | 0.975 | 0.5 |
��6min | 0.3 | 0.975 | 0.5 |
��8min | 0.7 | 1.475 | 0.1 |
�ٷ�Ӧ�ڵ�2min����4min�ڣ�O2��ƽ����Ӧ����Ϊ______��
�ڷ�Ӧ�ڵ�6minʱ�ı����������ı������������______(�����)��
A ʹ�ô���������B �����¶�C ��СѹǿD ����O2��Ũ��
������˵������˵��4NH3(g)+5O2(g)4NO(g)+6H2O(g)�ﵽƽ��״̬����_____(�����)��
A ��λʱ��������nmolNO��ͬʱ������nmolNH3
B ����һ������������ƽ����Է����������ٱ仯
C �ٷֺ���w(NH3)=w(NO)
D ��Ӧ����v(NH3)��v(O2) ��v(NO) ��v(H2O)=4��5��4��6
E ���ں��º�ѹ���ݻ��ɱ�������з�Ӧ�����������ܶȲ��ٱ仯
(3)ij�о�����װ��CH3OH-O2ȼ�ϵ�صĹ���ԭ����ͼ��ʾ��
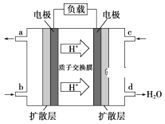
�ٸõ�ع���ʱ��b��ͨ�������Ϊ______��
�ڸõ�������ĵ缫��ӦʽΪ______��
���𰸡�-1168.8kJ/mol 0.1875mol/��Lmin�� B ABE CH3OH O2+4e-+4H+=2H2O
��������
(1)������ȼ����Ϊ285.8kJ/mol��д���Ȼ�ѧ��Ӧ����ʽ��������еķ���ʽ�����ݸ�˹���ɷ������
(2)�ٸ��ݰ�����Ũ�ȱ仯������������ʣ��������������ʣ�
���ȷ���ͼ���з�Ӧ����������Ũ�ȵı仯���ٸ������Ի�ѧƽ���Ӱ�������
�۸��ݴﵽƽ��״̬�����淴Ӧ������ȣ�����ֵ�Ũ�ȡ��ٷֺ������ٱ仯�������жϣ�
(3)����ͼʾ�������ӵ��ƶ���������жϸ�ȼ�ϵ�ص������������ԭ���ԭ���������
(1)��֪������ȼ����Ϊ285.8kJ/mol�����O2(g)+2H2(g)�T2H2O(l)��H=-571.6kJ/mol����N2(g)+3H2(g)�T2NH3(g)��H=-92.4kJ/mol����N2(g)+O2(g)�T2NO(g)��H=+180.6kJ/mol���ɸ�˹���ɣ�3����-2����+2���۵ã�4NH3(g)+5O2(g)4NO(g)+6H2O(l) ��H=(-571.6kJ/mol)��3-(-92.4kJ/mol)��2 +(+180.6kJ/mol)��2 =-1168.8kJ/mol���ʴ�Ϊ��-1168.8kJ/mol��
(2)�ٷ�Ӧ�ķ���ʽΪ4NH3(g)+5O2(g)4NO(g)+6H2O(l)��H=-1168.8kJ/mol��������ƽ����ѧ��Ӧ����Ϊv=![]() =
=![]() =0.15 mol/(Lmin)��ͬһ��ѧ��Ӧͬһʱ����ڣ������ʵķ�Ӧ����֮�ȵ��ڼ�����֮�ȣ�����������ƽ����ѧ��Ӧ����Ϊ��0.15 mol/(Lmin)��
=0.15 mol/(Lmin)��ͬһ��ѧ��Ӧͬһʱ����ڣ������ʵķ�Ӧ����֮�ȵ��ڼ�����֮�ȣ�����������ƽ����ѧ��Ӧ����Ϊ��0.15 mol/(Lmin)��![]() =0.1875 mol/(Lmin)���ʴ�Ϊ��0.1875mol/(Lmin)��
=0.1875 mol/(Lmin)���ʴ�Ϊ��0.1875mol/(Lmin)��
��ͨ��ͼ���Ƚϵ�6min�͵�8min��Ӧ����������Ũ��֪����Ӧ��Ũ������������Ũ�ȼ�С��ƽ�����ƣ����Ըı�������������¶ȣ���B��ȷ���ʴ�Ϊ��B��
��A����λʱ��������n mol NOΪ�����ʣ�����n molNH3Ϊ�����ʣ�������������֮�ȵ��ڻ�ѧ������֮�ȣ���ʾ���淴Ӧ������ȣ���˵����Ӧ�ﵽ��ƽ��״̬����A��ȷ��B���÷�Ӧ������������ʵ�������ķ�Ӧ��������������䣬������ƽ����Է���������С��������������ƽ��Ħ���������䣬˵�����淴Ӧ������ȣ��ﵽ��ƽ��״̬����B��ȷ��C���ٷֺ���w(NH3)=w(NO)������˵����Ӧ����������Ũ�Ȳ��ٱ仯���������ж��Ƿ�ﵽƽ��״̬����C����D����Ӧ����v(NH3)��v(O2) ��v(NO) ��v(H2O)=4��5��4��6����Ӧ��ʼ��ʼ�ճ���������˵����Ӧ�ﵽƽ��״̬����D����E���÷�Ӧ���������ķ�Ӧ����Ӧ������������������䣬����������ܶ���С�����ܶȲ����ˣ�˵����Ӧ�ﵽ��ƽ��״̬����E��ȷ���ʴ�Ϊ��ABE��
(3)�ٸ���ͼʾ����������a��缫����c��缫����c��缫Ϊ����������c��ͨ�������������b��ͨ�������ΪCH3OH���ʴ�Ϊ��CH3OH��
��c��缫Ϊ������ͨ����������������ĵ缫��ӦΪ��O2+4e-+4H+=2H2O���ʴ�Ϊ��O2+4e-+4H+=2H2O��

 ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�