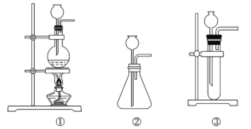
��Ŀ����
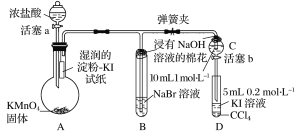
����Ŀ��ijѧϰС����ͼʾװ�òⶨ��þ�Ͻ������������������������ԭ��������
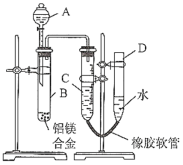
(1)A���Լ�Ϊ__________________
(2)ʵ��ǰ���Ƚ���þ�Ͻ���ϡ���н���Ƭ�̣���Ŀ����_______________________________
(3)��������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú�����еIJ������У��ټ�¼C��Һ��λ�ã��ڽ�B��ʣ�������ˣ�ϴ�ӣ�������أ��۴�B�в���������������ָ������º�¼C��Һ��λ�ã�����A��B�μ������Լ�������������˳����_______________________������ţ���
(4)B�з�����Ӧ�Ļ�ѧ����ʽΪ_____________________________________________________
(5)��ʵ������þ�Ͻ������Ϊag������������ΪbmL(�ѻ���Ϊ��״��)��B��ʣ����������Ϊcg�����������ԭ������Ϊ____________________��
���𰸡�NaOH��Һ ��ȥ���������Ĥ���ں�����Ӧ �٢ܢۢ� 2Al+2NaOH+6H2O��2NaAlO2+3H2�� ![]()
��������
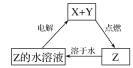
(1)��������ǿ�Ӧ��þ����Ӧ���ݴ˷�����
(2)ʵ��ǰ����������������Ĥ���Ƚ���þ�Ͻ���ϡ���н���Ƭ����Ŀ���dz�ȥ���������Ĥ���ں�����Ӧ��
(3)�ȼ�¼Һ�棬�ټ�������������Һ��ַ�Ӧ��ֱ�����ٲ���������ٽ�������ˣ�ϴ�Ӹ��
(4)B�������������Ʒ�Ӧ��
(5)���ݷ���ʽ�Ĺ�ϵ���м��㡣
(1)A���Լ�������Ӧ����þ����Ӧ�����������������Һ���ʴ�Ϊ������������Һ��
(2)ʵ��ǰ����������������Ĥ������Ƚ���þ�Ͻ���ϡ���н���Ƭ�̣���Ŀ���dz�ȥ���������Ĥ���ں�����Ӧ���ʴ�Ϊ����ȥ���������Ĥ���ں�����Ӧ��
(3)�ȼ�¼Һ�棬�ټ�������������Һ��ַ�Ӧ��ֱ�����ٲ���������ٽ�������ˣ�ϴ�Ӹ���������������˳�����٢ܢۢ����ʴ�Ϊ�٢ܢۢ���
(4)B�������������Ʒ�Ӧ��������Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+6H2O ��2NaAlO2 + 3H2�� ���ʴ�Ϊ��2Al+2NaOH+6H2O��2NaAlO2+3H2����
(5) 2Al+2NaOH+6H2O��2NaAlO2+3H2��
2xg 67.2L
(a-c)g b��10-3L
2xg �� (a-c)g = 67.2L��b��10-3L����� x = ![]() ����Al�����ԭ������Ϊ
����Al�����ԭ������Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�����Ŀ����ҵ���������Ҫ��ӦΪ4NH3(g)+5O2(g)4NO(g)+6H2O(l)��H
(1)��֪��������ȼ����Ϊ285.8kJmol-1
N2(g)+3H2(g)2NH3(g)��H=-92.4kJmol-1
N2(g)+O2(g)2NO(g)��H=+180.6kJmol-1
��������ҵ���������Ҫ��Ӧ����H=______��
(2)���ݻ��̶����ܱ������з���������Ӧ�������ڲ������ʵ����ʵ���Ũ�����±���
Ũ�� | c(NH3)(molL-1) | c(O2)(molL-1) | c(NO)(molL-1) |
��ʼ | 0.8 | 1.6 | 0 |
��2min | 0.6 | a | 0.2 |
��4min | 0.3 | 0.975 | 0.5 |
��6min | 0.3 | 0.975 | 0.5 |
��8min | 0.7 | 1.475 | 0.1 |
�ٷ�Ӧ�ڵ�2min����4min�ڣ�O2��ƽ����Ӧ����Ϊ______��
�ڷ�Ӧ�ڵ�6minʱ�ı����������ı������������______(�����)��
A ʹ�ô���������B �����¶�C ��СѹǿD ����O2��Ũ��
������˵������˵��4NH3(g)+5O2(g)4NO(g)+6H2O(g)�ﵽƽ��״̬����_____(�����)��
A ��λʱ��������nmolNO��ͬʱ������nmolNH3
B ����һ������������ƽ����Է����������ٱ仯
C �ٷֺ���w(NH3)=w(NO)
D ��Ӧ����v(NH3)��v(O2) ��v(NO) ��v(H2O)=4��5��4��6
E ���ں��º�ѹ���ݻ��ɱ�������з�Ӧ�����������ܶȲ��ٱ仯
(3)ij�о�����װ��CH3OH-O2ȼ�ϵ�صĹ���ԭ����ͼ��ʾ��
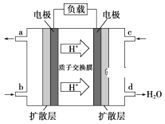
�ٸõ�ع���ʱ��b��ͨ�������Ϊ______��
�ڸõ�������ĵ缫��ӦʽΪ______��