��Ŀ����
(17��)���û�ѧ��Ӧԭ���о�̼�Ļ����������Ҫ���塣
��1�������� �����ڼ��CO����Ӧԭ��Ϊ��
�����ڼ��CO����Ӧԭ��Ϊ��
 ����2L�ܱ������м�������
����2L�ܱ������м������� ����ͨ��1molCO��CO2���������
����ͨ��1molCO��CO2��������� ��ʱ��ı仯����ͼ��ʾ��
��ʱ��ı仯����ͼ��ʾ��

��0��0.5min�ڵ�ƽ����Ӧ���� _____________��
_____________��
�ڱ����¶Ⱥ�������䣬����ʼ����CO(g)�����ʵ�����ԭ����2����������˵����ȷ����____________(�����)��
a������I2������Ϊԭ����2��
b����������ƽ��Ħ����������
c���ﵽƽ���ʱ��Ϊԭ����2��
d�����������ܶȲ���
�۷�Ӧ��a��ʱ�������������� ���������
��������� ���ɲ�ȡ�Ĵ�ʩΪ____________��
���ɲ�ȡ�Ĵ�ʩΪ____________��
��2���� Ϊ���������Խ�
Ϊ���������Խ� �Ļ������ֱ��ת��Ϊ���ᡣ
�Ļ������ֱ��ת��Ϊ���ᡣ
�����÷�Ӧ��ԭ��������Ϊ100������ ______________��
______________��
����25���£���pH=a������������Һ��pH=b�Ĵ�����Һ�������ϣ�������Һǡ����ȫ��Ӧ���� ________14(�>������<����=��)�����¶��´���ĵ��볣��K=__________(�ú�a��b��ʽ�ӱ�ʾ)��
________14(�>������<����=��)�����¶��´���ĵ��볣��K=__________(�ú�a��b��ʽ�ӱ�ʾ)��
��3�����÷�Ӧ ���Դ�������β���������÷�Ӧ���Ϊԭ��أ�������Na2O������ʣ��������缫��ӦʽΪ________________________________��
���Դ�������β���������÷�Ӧ���Ϊԭ��أ�������Na2O������ʣ��������缫��ӦʽΪ________________________________��
��1��������
 �����ڼ��CO����Ӧԭ��Ϊ��
�����ڼ��CO����Ӧԭ��Ϊ��
 ����2L�ܱ������м�������
����2L�ܱ������м������� ����ͨ��1molCO��CO2���������
����ͨ��1molCO��CO2��������� ��ʱ��ı仯����ͼ��ʾ��
��ʱ��ı仯����ͼ��ʾ��
��0��0.5min�ڵ�ƽ����Ӧ����
 _____________��
_____________���ڱ����¶Ⱥ�������䣬����ʼ����CO(g)�����ʵ�����ԭ����2����������˵����ȷ����____________(�����)��
a������I2������Ϊԭ����2��
b����������ƽ��Ħ����������
c���ﵽƽ���ʱ��Ϊԭ����2��
d�����������ܶȲ���
�۷�Ӧ��a��ʱ��������������
 ���������
��������� ���ɲ�ȡ�Ĵ�ʩΪ____________��
���ɲ�ȡ�Ĵ�ʩΪ____________����2����
 Ϊ���������Խ�
Ϊ���������Խ� �Ļ������ֱ��ת��Ϊ���ᡣ
�Ļ������ֱ��ת��Ϊ���ᡣ�����÷�Ӧ��ԭ��������Ϊ100������
 ______________��
______________������25���£���pH=a������������Һ��pH=b�Ĵ�����Һ�������ϣ�������Һǡ����ȫ��Ӧ����
 ________14(�>������<����=��)�����¶��´���ĵ��볣��K=__________(�ú�a��b��ʽ�ӱ�ʾ)��
________14(�>������<����=��)�����¶��´���ĵ��볣��K=__________(�ú�a��b��ʽ�ӱ�ʾ)����3�����÷�Ӧ
 ���Դ�������β���������÷�Ӧ���Ϊԭ��أ�������Na2O������ʣ��������缫��ӦʽΪ________________________________��
���Դ�������β���������÷�Ӧ���Ϊԭ��أ�������Na2O������ʣ��������缫��ӦʽΪ________________________________����1����0.8mol/(L��min) ��ab �۽����¶�
��2����2 �ڣ�
��3��2NO2+8e-=N2+4O2-
��2����2 �ڣ�

��3��2NO2+8e-=N2+4O2-
�����������1������Ϊ������ӦΪ�������ǰ��ķ�Ӧ��0.5minʱCO2���������Ϊ0.80����CO2�����ʵ���Ϊ0.8mol����CO�����ʵ����仯��Ҳ��0.8mol��0��0.5min�ڵ�ƽ����Ӧ����

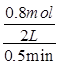 =0.8mol/(L��min)���ڱ����¶Ⱥ�������䣬����ʼ����CO(g)�����ʵ�����ԭ����2����������I2������Ϊԭ����2��������������������ԭ����2���������ʵ�����ԭ����2����ƽ��Ħ���������䣬�ܶ���ԭ����2����Ũ�������·�Ӧ��������ƽ���ʱ����٣���ab��ȷ���۷�Ӧ��a��ʱ��������������
=0.8mol/(L��min)���ڱ����¶Ⱥ�������䣬����ʼ����CO(g)�����ʵ�����ԭ����2����������I2������Ϊԭ����2��������������������ԭ����2���������ʵ�����ԭ����2����ƽ��Ħ���������䣬�ܶ���ԭ����2����Ũ�������·�Ӧ��������ƽ���ʱ����٣���ab��ȷ���۷�Ӧ��a��ʱ�������������� ���������
��������� ����ʹƽ��������Ӧ�����ƶ�����÷�ӦΪ���ȷ�Ӧ���ʿɽ����¶ȡ�
����ʹƽ��������Ӧ�����ƶ�����÷�ӦΪ���ȷ�Ӧ���ʿɽ����¶ȡ���2����������COx+CH4��CH3COOH�����÷�Ӧ��ԭ��������Ϊ100���������Ԫ���غ㣬x=2����pH=a������������Һ��pH=b�Ĵ�����Һ�������ϣ�������Һǡ����ȫ��Ӧ����˵��c(CH3COOH)=c(NaOH)=10-(14-a)mol/L������pH=b�����Դ�����������Ũ��Ϊ10-bmol/L������Ϊ���ᣬ����̶�С��1����10-b/10-(14-a)��1��a+b��14�����¶��´���ĵ��볣��K=
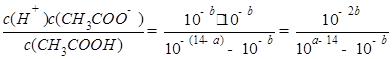 ��
����3��ԭ�������������ԭ��Ӧ�������ܷ�Ӧ��֪NO2�õ��ӱ���ԭ�����ɵ������ʵ缫��ӦΪ��2NO2+8e-=N2+4O2-��

��ϰ��ϵ�д�
�����Ŀ
 XC��g������ 2 s��Ӧ��ƽ��,��� C ��Ũ��Ϊ 0.6 mol��L��1 ��B�����ʵ���Ϊ1.4 mol,�������м���˵����
XC��g������ 2 s��Ӧ��ƽ��,��� C ��Ũ��Ϊ 0.6 mol��L��1 ��B�����ʵ���Ϊ1.4 mol,�������м���˵����  CH3OH+H2O����ش��������⣺
CH3OH+H2O����ش��������⣺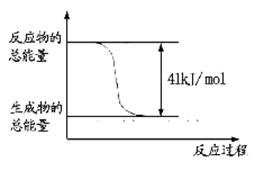
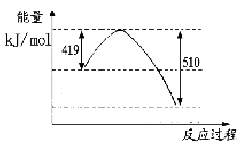 д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ__ _��
д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ__ _��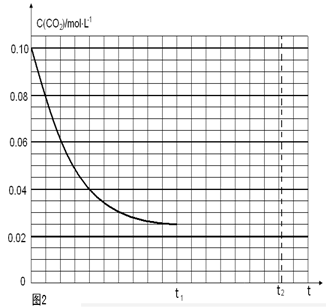

 2SO3(g)����H��0
2SO3(g)����H��0
 AB��˵�������°�����������з�Ӧ��
AB��˵�������°�����������з�Ӧ�� 4NO(g)+ 6H2O(g)������������ȷ����
4NO(g)+ 6H2O(g)������������ȷ����