��Ŀ����
����ͼʾ���Ӧ�������������
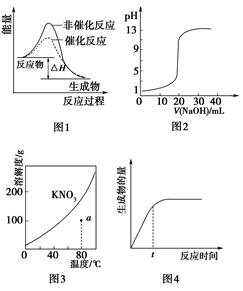

| A��ͼ1��ʾij���ȷ�Ӧ�ֱ����С�����������·�Ӧ�����е������仯 |
| B��ͼ2��ʾ0.1000 mol��L��1NaOH��Һ�ζ�20.00 mL 0.1000 mol��L��1CH3COOH��Һ���õ��ĵζ����� |
| C��ͼ3��ʾKNO3���ܽ�����ߣ�ͼ��a����ʾ����Һ��80 ��ʱKNO3�IJ�������Һ |
| D��ͼ4��ʾij���淴Ӧ����������淴Ӧʱ��仯�����ߣ���ͼ֪tʱ�̷�Ӧ��ת������� |
C
���������A��ͼ�з�Ӧ������������������������Ϊ���ȷ�Ӧ������B������20ml NaOH��Һ��ǡ����ȫ��Ӧ������CH3COONa��ˮ���Լ��ԣ�pH������7������C��KNO3���ܽ�����¶ȵ����߶�����a�����������£�˵��û�дﵽ���ͣ���ȷ��D��tʱ�̻�û�е���ƽ��״̬����Ӧ��ת���ʲ��������

��ϰ��ϵ�д�
�����Ŀ
 AsO33����I2��H2O����Ƴɵ�ԭ���װ�ã�����C1��C2��Ϊ̼����������ͼ���ձ�����μ�������Ũ���������ͼ��B�ձ�����μ�������40%NaOH��Һ��
AsO33����I2��H2O����Ƴɵ�ԭ���װ�ã�����C1��C2��Ϊ̼����������ͼ���ձ�����μ�������Ũ���������ͼ��B�ձ�����μ�������40%NaOH��Һ��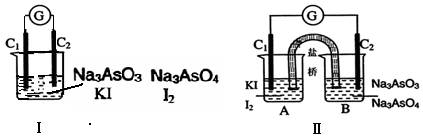
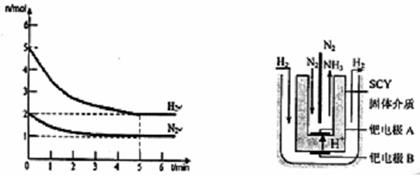
 2NH3(g)����������������ʱ��ѹ���������ʹѹǿ��������Ӧ���淴Ӧ�����Լ�H2��ƽ��ת���ʾ�����
2NH3(g)����������������ʱ��ѹ���������ʹѹǿ��������Ӧ���淴Ӧ�����Լ�H2��ƽ��ת���ʾ�����



 ��������������ʱ�����������Ӧ����v��SO2����SO2ת���ʾ�����
��������������ʱ�����������Ӧ����v��SO2����SO2ת���ʾ�����



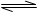 2NH3��g���ġ�H= ��
2NH3��g���ġ�H= ��