��Ŀ����
1�����������ܴ���������ǣ�������| A�� | ʹ��̪��Һ������Һ��Na+��K+��SO${\;}_{4}^{2-}$��HCO${\;}_{3}^{-}$ | |
| B�� | ��ɫ������Һ�У�MnO${\;}_{4}^{-}$��K+��SO${\;}_{4}^{2-}$��Na+ | |
| C�� | ���д���Mg2+����Һ�У�Fe2+��SO${\;}_{4}^{2-}$��NO${\;}_{3}^{-}$��Cl- | |
| D�� | ʹ��ɫʯ����Һ������Һ��NH${\;}_{4}^{+}$��NO${\;}_{3}^{-}$��Al3+��CH3COO- |
���� A��ʹʹ��̪��Һ������Һ�ʼ��ԣ�̼��������������������ӷ�Ӧ��
B����ɫ������Һ�в�������ɫ�ĸ���������ӣ�
C����������֮�䲻��Ӧ��������þ���ӷ�Ӧ��
D��ʹ��ɫʯ����Һ������ҺΪ������Һ������������������ӷ�Ӧ���ɴ��ᣮ
��� �⣺A����̪��Һ������Һ�д��ڴ������������ӣ�HCO3-�����������ӷ�Ӧ������Һ�в��ܴ������棬��A����
B��MnO4-Ϊ��ɫ���ӣ���������Һ��ɫ����������B����
C��Fe2+��SO42-��NO3-��Cl-֮�䲻������Ӧ��������Mg2+��Ӧ������Һ���ܹ��������棬��C��ȷ��
D��ʹ��ɫʯ����Һ������Һ�д��ڴ��������ӣ�CH3COO-�������ӽ�����ɴ��ᣬ����Һ�в��ܴ������棬��D����
��ѡC��
���� ���⿼�������ӹ�����жϣ���Ŀ�Ѷ��еȣ���ȷ���ӷ�Ӧ����������Ϊ���ؼ���ע�����а��������ĺ��壬����������ѧ���ķ������������������Ӧ��������

��ϰ��ϵ�д�
 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
�����Ŀ
9�������й�Ԫ�������ɵ���������ȷ���ǣ�������
| A�� | �����ԣ�F2��Cl2 | B�� | ��̬�⻯����ȶ��ԣ�H2O��H2S | ||
| C�� | ���ԣ�H3PO4��H2SO4 | D�� | ���Ӱ뾶��Na+��Na |
16�����з�Ӧ�У��ǽ�������δ���ֳ������Ե��ǣ�������
| A�� | ������ǿ����Һ���� | B�� | ����������ȼ�� | ||
| C�� | ���������з�Ӧ�������� | D�� | ���ڿ�����ȼ�� |
6�����й���������ԭ��Ӧ��������ȷ���ǣ�������
| A�� | H2��ԭCuO�ķ�Ӧֻ���ڻ�ԭ��Ӧ | |
| B�� | �û���Ӧ����������ԭ��Ӧ������3CO+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2�����û���Ӧ����������ԭ��Ӧ | |
| C�� | 3O2$\frac{\underline{\;���\;}}{\;}$2O3��������ԭ��Ӧ | |
| D�� | ���ֽⷴӦһ������������ԭ��Ӧ |
13�����з�Ӧ�У���˵���ȵ������Ա���ǿ�ķ�Ӧ�ǣ�������
| A�� | CuCl2+H2S�TCuS��+2HCl | B�� | 2HCl+Na2S�T2NaCl+H2S | ||
| C�� | Cl2+H2S�T2HCl+S | D�� | 3Cl2+6FeSO4�T2Fe2��SO4��3+2FeCl3 |
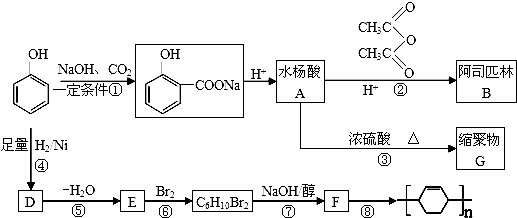
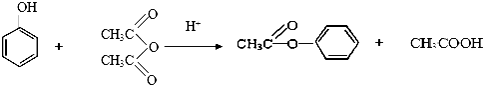
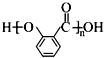 ���ݵķ�Ӧ������NaOH�Ĵ���Һ�����ȣ���ķ�Ӧ�����ǼӾ۷�Ӧ��
���ݵķ�Ӧ������NaOH�Ĵ���Һ�����ȣ���ķ�Ӧ�����ǼӾ۷�Ӧ��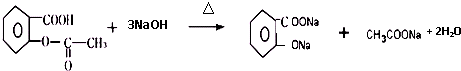 ��
��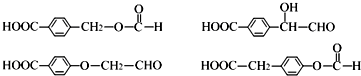 ��
��