��Ŀ����
����Ŀ����25��ʱ��̼�����ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪25��ʱ����Ƶ�Ksp=9.1��10-6������˵������ȷ����
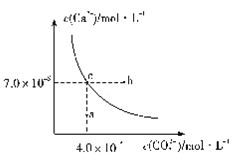
A. ��ȥ��¯ˮ��������Ƶİ취�ǽ���ת��Ϊ̼��ƣ�Ȼ������ȥ��
B. ͼ��b��̼��ƵĽᾧ���ʴ������ܽ�����
C. ͨ����������ʹ��Һ��a��仯��c��
D. ��25��ʱ����ӦCaSO4(s)+CO32-(aq)===CaCO3(s)+SO42-(aq)��ƽ�ⳣ��K=3250
���𰸡�C
��������A. ����ͼ���֪̼��Ƶ��ܶȻ�������7��10��6��4��10��4��2.8��10��9��С������Ƶ��ܶȻ���������˳�ȥ��¯ˮ��������Ƶİ취�ǽ���ת��Ϊ̼��ƣ�Ȼ������ȥ����A��ȷ��B. ͼ��b���������Ϸ����ǹ�������Һ�����̼��ƵĽᾧ���ʴ������ܽ����ʣ�C��ȷ��C. ͨ����������Һ�����С�������Ӻ�̼������ӵ�Ũ�Ⱦ�������ʹ��Һ��a��仯��c�㣬C����D. ��25��ʱ����ӦCaSO4(s)+CO32-(aq)===CaCO3(s)+SO42-(aq)��ƽ�ⳣ��K=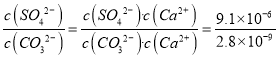 ��3250��D��ȷ����ѡC��
��3250��D��ȷ����ѡC��

����Ŀ��300��ʱ��������X������Y��0.16mol����10L�����ܱ������У�������Ӧ��
X(g)+Y(g)![]() 2Z(g) ��H<0��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ���������±���
2Z(g) ��H<0��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ���������±���
t/min | 2 | 4 | 7 | 9 |
n(Y)/mol | 0.12 | 0.11 | 0.10 | 0.10 |
����˵����ȷ���� ( )
A. ǰ2min��ƽ����Ӧ����v(X)=2.0��10-2mol/(L��min)
B. �����������䣬�ٳ���0.1 mol X��0.1mol Y���ٴ�ƽ��ʱY��ת���ʲ���
C. ��v��(Y)=2v��(Z)ʱ��˵����Ӧ�ﵽƽ��
D. �÷�Ӧ��250��ʱ��ƽ�ⳣ��С��1.44
