��Ŀ����
����Ŀ����¼Ƭ�����ڹʹ������չʾ��ר�Ҿ�տ�ļ��պͶԴ�ͳ�Ļ����Ȱ�����أ�Ҳ������ᵽ��ѧ���������ﱣ���еľ����á�
��.ij����������������Ĺ������£�
(1)�����ʴ��ijɷ֡�
��ʴ����Ҫ�ɷֵĻ�ѧʽ | |||
Fe3O4 | Fe2O3��H2O | FeO(OH) | FeOCl |
FeOCl����Ԫ�صĻ��ϼ�Ϊ_______��
(2)��ѧ�����������ȡ���ԭ���γ�Fe3O4�����㡣(��֪��Cl����ӿ����ĸ�ʴ)
�����̣���������û��ʢ��0.5 mol��L-1 Na2SO3��0.5 mol��L-1 NaOH��Һ�������У�����������60��90�棬һ��ʱ���ȡ�������NaOH��Һϴ������Cl����
�����ȷ�Ӧ��FeOCl + OH�� = FeO(OH) + Cl�������ӷ�Ӧ����������Ũ�ȼ�С�ķ�����еģ�����ͬ�¶��£�FeOCl��FeO(OH)���ܽ�ȣ�s(FeOCl)______s[FeO(OH)](�������)��
�ڻ�ԭ��Ӧ��Na2SO3��ԭFeO(OH)�γ�Fe3O4����ƽ��ѧ����ʽ��
___Na2SO3 + ___FeO(OH) = ___Na2SO4 + ___Fe3O4 + ___H2O
�ۼ���Cl���Ƿ�ϴ�Ӹɾ��ķ�����_______________��
��.��Ҫ����500mL 0.5mol��L-1��NaOH��Һ����ϴ������ش��������⣺
(3)����ʱ��Ӧ����ƽ��ȡNaOH������Ϊ___________(��ȷ��С�������λ)��
(4)ij����������ͼ��ʾ��
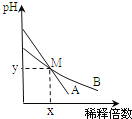
�ò���Ӧ������ͼ��ʾ��____________(�����)����֮�䡣
�� ��
��![]() ��
��![]() ��
��![]() ��
�� ��
��![]()
(5)����˵���������_____________(����)��
A.����NaOH�����ʱ�䲻�˹���
B.���ձ�����Һ��ȴ�����²Ž���ת��
C.����ʱ����������ƿ�̶���ʹ���Ƶ�NaOH��ҺŨ��ƫ��
D.����ƿ�ɴ����õ�NaOH��Һ
���𰸡�+3 �� 1 6 1 2 3 ȡ�������һ��ϴ��Һ���Թ��У�����ϡ��������ҺΪ���ԣ��ټ���������Һ�����ް�ɫ��������˵����ϴ�Ӹɾ� 10.00 g �ܢ� CD
��������
(1)���ݻ������и�Ԫ�ػ��ϼ۽��з������㣻
(2)�������ܽ�Ƚ��з�����
�ڸ��ݻ��ϼ�������ȣ������غ���д���ӷ���ʽ��
�����������ữ�����������飻
��3������m=cVM���м��㣻
(4)���ݲ���������з�����
(5)��������һ��Ũ����Һ������жϡ�
(1) FeOCl��OԪ��Ϊ-2�ۣ�ClԪ��Ϊ-1�ۣ����ݻ�����Ļ��ϼ۴����ܺ�Ϊ0�ɵ���Ԫ�صĻ��ϼ�Ϊ+3�ۣ�
(2)����Ϊ���ӷ�Ӧ�ı���������Ũ�ȵļ�С�����s(FeOCl)��s[FeO(OH)]��
�ڸ��ݻ��ϼ�������ȣ������غ���д���ӷ���ʽΪNa2SO3 +6FeO(OH) = Na2SO4 + 2Fe3O4 + 3H2O��
�ۼ��ϴ��Һ��Cl���ķ�����ȡ����ϴ��Һ���Թ��У�����ϡ��������ҺΪ���ԣ��ټ���������Һ�����ް�ɫ��������˵����ϴ�Ӹɾ���
��3�����������Ƶ�����Ϊm=0.5L��0.5molL-1��40g/mol=10.0g��
(4)ͼ�в����Ƕ��ݣ�����Ӧ��תҺ��ҡ��֮�䣬��Ӧ����ʾ�Ģܢݲ���֮�䣻
(5)A.��NaOH������ˮ����¶�ڿ����г���NaOH�����ʱ��̫�������ʵ�ʳƵõ�������������ƫС��������Һ��Ũ��ƫ�ͣ�ѡ��A��ȷ��
B. ĩ��ȴ�����¾�ת�ƽ�����ƿ���ᵼ����Һ�����ƫС����Һ��Ũ��ƫ�ʴ��ձ�����Һ��ȴ�����²Ž���ת�ƣ�ѡ��B��ȷ��
C.����ʱ����������ƿ�̶��ߣ�������Һ���ƫ��ʹ���Ƶ�NaOH��ҺŨ��ƫ�ͣ�ѡ��C����
D. ����ƿ�������ڱ�����ڴ��NaOH��Һ��ѡ��D����
��ѡCD��

����Ŀ��
�������̼��Ԫ���ڿ���������������������й㷺��Ӧ�á��ش��������⣺
(1)��Ԫ�����ڱ��У���Li�Ļ�ѧ���������Ƶ�����Ԫ����__________(��Ԫ�ط���)����Ԫ�ػ�̬ԭ���������ӵ�����״̬___________(������ͬ�������෴��)��
(2)̼����йػ�ѧ������������ʾ��
��ѧ�� | C-H | C-O | Si-H | Si-O |
����/kJmol-1 | 413 | 336 | 318 | 452 |
SiH4���ȶ���С��CH4���������������ԭ����__________________��
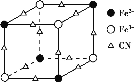
(3)��Ȼ�����ζ�����[SiO4]�������Զ�����ԭ���������ɣ��ɳ���״Ҳ�ɳɻ������Թ���������ࡣ��ͼa����SiO44-��b��c�ǹ����������γɵĻ�״�ṹ��
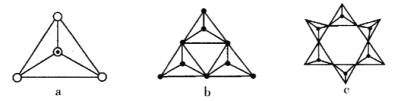
������������Si�Ĺ���ӻ�����Ϊ____________�� ͼb��״�ṹ������Ļ�ѧʽΪ______________���ڻ�״�ṹ�й��ԭ����Ϊn��д����״�ṹ�й������ͨʽ_____________��
(4)�������������γ��廯�ؾ��壬�þ�������Ϊ___________���侧���ܿ�ͨ����ͼ��Borm-Haberѭ������õ���
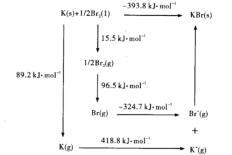
����ͼ��֪��Kԭ�ӵĵ�һ������Ϊ_____ kJ/mol�� Br-Br������Ϊ______kJ/ mol��KBr�ľ�����Ϊ______kJ/mol��������Խ�þ�����۵�Խ______��