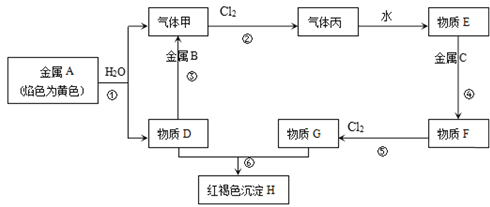
��Ŀ����
����Ŀ����֪25 ��ʱ����������ʵĵ���ƽ�ⳣ�����±���ʾ��
��ѧʽ | CH3COOH | H2CO3 | HClO | |
ƽ�ⳣ�� | Ka=1.8��10-5 | Ka1=4.3��10-7 | Ka2=5.6��10-11 | Ka=3.0��10-8 |
�ش��������⣺
��1�����ʵ���Ũ�Ⱦ�Ϊ0.1 mol��L-1��������Һ��
a. CH3COOH������ b. H2CO3 c. NaHCO3 d. HClO
pH��С���������˳����____(����ĸ)��
��2�������£�0.1 mol��L-1CH3COOH��Һ��ˮϡ�����У����б���ʽ����ֵ������____(����ĸ)��
A. c(H+) B.![]() C. c(H+)��c(OH-) D.
C. c(H+)��c(OH-) D.![]() E.
E.![]()
��3�������Ϊ100 mL��pH=2��CH3COOH��һԪ��HX����ˮϡ������pH����Һ����Ĺ�ϵ����ͼ��ʾ����HX�ĵ���ƽ�ⳣ��____(����ڡ�����С�ڡ����ڡ�)CH3COOH�ĵ���ƽ�ⳣ����������____�������������pH����ͬ��CH3COOH��һԪ��HX�м���������п�����ɵ���������ͬ����������С��ϵΪ��CH3COOH____(����ڡ�����С�ڡ����ڡ�)HX

���𰸡�a<b<d<c BD ���� ��ˮϡ����ͬ����HX��pH�仯������ǿ������ƽ�ⳣ���� С��
��������
��1����ͬ���ʵ���Ũ�ȵ�������Һ���������ˮ��̶�Խ������Һ��pHԽ��
��2��CH3COOH��Һ��ˮϡ���̣��ٽ����룬c��H+����С��c��OH-������Kw���䣻
��3����ͼ��֪��ϡ����ͬ�ı�����HX��pH�仯�̶ȴ�������HXǿ������ƽ�ⳣ����
��1���ɵ���ƽ�ⳣ���ж����Ե�ǿ��������Խǿ�����Ӧ�ε�ˮ��̶�Խ����Һ��pH��Խ���ɱ����е����ݿ�֪������CH3COOH��H2CO3��HClO��HCO3-�����������Խ����������ӵ�ˮ��̶�Խ����Һ�ļ���Խǿ������pH�ɴ�С��˳����a��d��c��b���ʴ�Ϊ��a��d��c��b��
��2��A.CH3COOH��Һ��ˮϡ���̣��ٽ����룬c��H+����С����A��ѡ��
B.c��H+��/c��CH3COOH��=n��H+��/n��CH3COOH������ϡ�����б�ֵ���Bѡ��
C.ϡ���̣��ٽ����룬c��H+����С��c��OH-������c��H+��c��OH-��=Kw��Kw���䣬��C��ѡ��
D.ϡ���̣��ٽ����룬c��H+����С��c��OH-��������c��OH-��/c��H+�����Dѡ��
E.����ĵ���ƽ�ⳣ�����䣬��E��ѡ��
�ʴ�Ϊ��BD��
��3��ϡ����ͬ�ı�����pH�仯���������ǿ����ͼ��֪����ˮϡ�͵Ĺ����У�HX��pH�仯�ȽϿ죬˵��HX�����Աȴ��������ǿ��HX�ĵ���ƽ�ⳣ���ȴ����pH��ͬ��һԪ�ᣬ��Խ�����Ũ��Խ����ͬ�����ͬpH�IJ�ͬһԪ��������п��Ӧ����Խ�����ɵ��������Խ������HX�����Աȴ��������ǿ���������ɵ���������ͬ����������HX��CH3COOH���ʴ�Ϊ�����ڣ���ˮϡ����ͬ����HX��pH�仯������ǿ������ƽ�ⳣ����С�ڡ�