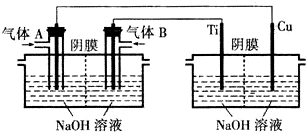
��Ŀ����
9�����װ����ͼ��ʾ��ͼ��װ����B��1L 2mol/L��AgNO3��Һ��Aװ������1L 2mol/L��Na2SO4��Һ��ͨ���ʪ��ĵ���KI��ֽ��X�˱���ɫ�����һ��ʱ����Իش���1��Ag�ǵ��ص�������д���˵缫�ĵ缫����ʽ��Ag-e=Ag+��
��2��A�з�����Ӧ�Ļ�ѧ����ʽΪ2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2+O2��
��3��B�й۲쵽������Ag����ϸ��Fe����������ɫ����������
��4�������£������һ��ʱ����õ�·��ͨ���ĵ��ӵ����ʵ���Ϊ0.2mol���ʵ������У�B������������21.6�ˣ�A�������ռ������������Ϊ1.12L����״������
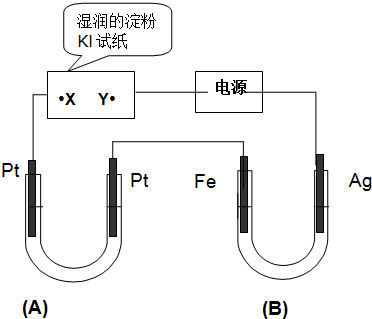
���� ����װ�ÿ�֪A��B��ʪ��ĵ���KI��ֽ�����ǵ��أ�ͨ���ʪ��ĵ���KI��ֽ��X�˱���ɫ˵���˵缫�Ϸ������ǵ�����ʧ����������I2���ж�XΪ������YΪ������B��Fe�缫Ϊ������AgΪ��������X������Pt�缫Ϊ��������Fe������Pt�缫Ϊ������
��1��AgΪ���������ʱAgʧ���ӷ���������Ӧ��
��2��Aװ������1L 2mol/L��Na2SO4��Һ�����Ե缫���ʵ�ʵ�����ˮ��������������������������������
��3��Bװ�õ�����AgΪ����ʧ���ӷ���������Ӧ���缫��ϸ��B��1L 2mol/L��AgNO3��Һ����Һ��Ag+����������ǿ���ȵõ����ӷ�����ԭ��Ӧ��Fe�缫����Ag��
��4����д�缫��Ӧʽ����ϵ����غ���������B��Fe�缫��������ΪAg���缫��ӦΪ��Ag++e-=Ag��A�������缫��ӦΪ4OH--4e-=2H2O+O2����
��� �⣺����װ�ÿ�֪A��B��ʪ��ĵ���KI��ֽ�����ǵ��أ�ͨ���ʪ��ĵ���KI��ֽ��X�˱���ɫ˵���˵缫�Ϸ������ǵ�����ʧ����������I2���ж�XΪ������YΪ������B��Fe�缫Ϊ������AgΪ��������X������Pt�缫Ϊ��������Fe������Pt�缫Ϊ������
��1������������֪AgΪ���������ʱAgʧ���ӷ���������Ӧ���缫��ӦΪ��Ag-e=Ag+��
�ʴ�Ϊ������Ag-e=Ag+��
��2��Aװ������1L 2mol/L��Na2SO4��Һ�����Ե缫���ʵ�ʵ�����ˮ����������������������������������Ӧ�Ļ�ѧ����ʽΪ��2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2 ����
�ʴ�Ϊ��2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2 ����
��3��Bװ�õ�����AgΪ����ʧ���ӷ���������Ӧ���缫��ϸ��B��1L 2mol/L��AgNO3��Һ����Һ��Ag+����������ǿ���ȵõ����ӷ�����ԭ��Ӧ��Fe�缫����Ag��Bװ���е�����Ϊ��Ag����ϸ��Fe����������ɫ����������
�ʴ�Ϊ��Ag����ϸ��Fe����������ɫ����������
��4�������£������һ��ʱ����õ�·��ͨ���ĵ��ӵ����ʵ���Ϊ0.2mol���������У�B��Fe�缫��������ΪAg���缫��ӦΪ��Ag++e-=Ag�������غ����õ�����Ag����=0.2mol��108g/mol=21.6g��A���ռ�������������������2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2 ����A�������缫��ӦΪ4OH--4e-=2H2O+O2��������ת��0.2mol����������O2���ʵ���=0.5mol����״�����������=0.5mol��22.4L/mol=1.12L��
�ʴ�Ϊ��21.6��1.12L��
���� �����ۺϿ�����ԭ���������ڿ���ѧ���ۺ����õ��֪ʶ����������Ŀ�Ѷ��еȣ�ע����յ����ɣ�����ȷ�жϵ缫����ʽ��

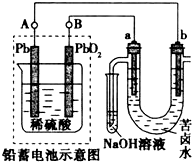 ��Ǧ���ص���±ˮ����C1-��Br-��Na+��Mg2+����װ����ͼ��ʾ��a��bΪʯī�缫��������˵������ȷ���ǣ�������
��Ǧ���ص���±ˮ����C1-��Br-��Na+��Mg2+����װ����ͼ��ʾ��a��bΪʯī�缫��������˵������ȷ���ǣ�������| A�� | Ǧ���طŵ�ʱ���õ��������������С | |
| B�� | Ǧ���س��ʱ��B��Ӧ�����Դ�������� | |
| C�� | ����±ˮʱ��a�缫���ȷŵ����Cl-������Br-����ΪCl-�Ļ�ԭ��ǿ��Br- | |
| D�� | ��b������0.01 mol ����ʱ��Ǧ����������0.02 molH2SO4 |
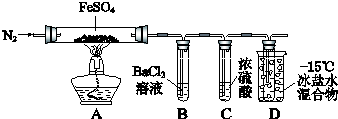
��֪��SO2�۵�-72�棬�е�-10�棻SO3�۵�16.8�棬�е�44.8�森
| ʵ����� | ʵ������ | |
| �� | ͨ��һ��ʱ��N2������ | A�й����Ϊ����ɫ��B���а�ɫ������D�Թ�������ɫҺ�� |
| �� | �ô��л��ǵ�ľ������װ��D�ĵ��ܿ� | ľ����ȼ |
| �� | ��ַ�Ӧ��ֹͣ���ȣ���ȴ��ȡA�й��壬������ | �����ܽ⣬��Һ�ʻ�ɫ |
| �� | ����������Һ����D�Թ��� | ��Һ��Ϊdz��ɫ |
��2��ʵ��۷�Ӧ�����ӷ���ʽ��Fe2O3+6H+=2Fe3++3H2O��
��3���ֽ���̳�����ʹľ����ȼ�������⣬����A�й�����ɫ�仯�Ʋ⣬��һ����SO2���壮��������Ϊ��Fe2O3���ɣ���FeSO4��ֻ��+6��SԪ���������ԣ��ܱ���ԭ�����һ����SO2���ɣ�
��4��ʵ��ܷ�Ӧ�����ӷ���ʽ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+��
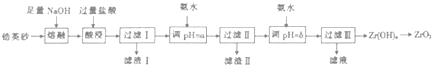
��֪����ZrO2�����ռӦ���ɿ�����ˮ��Na2ZrO2��Na2ZrO2���ᷴӦ����ZrO2+
�ڲ���������ʵ�������¿�ʼ��������ȫ����ʱ��pH���±�•
| ���� | Fe3+ | AP+ | ZrO2+ |
| ��ʼ����pH | 1.9 | 3.3 | 6.2 |
| ���ڳ���pH | 3.2 | 5.2 | 8.0 |
��2������I����Ҫ�ɷֵ�����Ϊ����
��3������ˢ�������Һ�мӰ�ˮ��pH=a����Ŀ���dz�ȥFe3+��Al3+Ȼ������Ӱ�ˮ��pH=b�ķ�Χ��b��8.0
��4������ˢ�������Һ�м���CaCO2��ĩ�����ȣ��ɵ�CO2����һ�����壬�÷�Ӧ�����ӷ���ʽΪ2NH4++CaCO3$\frac{\underline{\;\;��\;\;}}{\;}$Ca2++2NH3��+CO2��+H2O��
| A�� | ��һ���������к��ȵIJⶨʵ�飬������Ҫʹ���¶ȼƲ�4������ | |
| B�� | �������ظ������Һ��������NaOH���壬��Һ��ɫ�ɳ�ɫ������Ϊ��ɫ | |
| C�� | �ֱ�����֧ʢ��Ũ����ȵĴ���ͱ���������Һ�еμӵ�Ũ�ȵ�Na2CO3��Һ���۲쵽ǰ�߲������ݲ�����߾��� | |
| D�� | ��0.01mol/L��KMnO4��Һ�м���0.1mol/L��H2C2O4��Һ��ʵ�鿪ʼʱ��Һ��ɫ�ܿ죬������������Ҫ��������ҺŨ�ȱ仯���� |
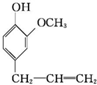 �����������ƿ���ܰ���㾫�Լ����춡��Ӻ������صȣ��ϳɶ�����ӵ�һ��·�����£�
�����������ƿ���ܰ���㾫�Լ����춡��Ӻ������صȣ��ϳɶ�����ӵ�һ��·�����£�
 ��X����±��ԭ�ӣ���
��X����±��ԭ�ӣ��� ��
�� ��
�� ��
��