��Ŀ����
����Ŀ��ijѧ����0.2000mol/L�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ���������: ���ñ���Һ��ϴ�ζ���2-3 �Σ�ȡ��NaOH ��Һע���ʽ�ζ�������0���̶������������̶��õζ��ܲ�ʹ�ζ��ܼ������Һ����������Һ������0������0���̶������£������¶���������ȡ20.00mL����Һע���徻����ƿ�У�������3�η�̪��Һ�����ñ�Һ�ζ����յ������µζ���Һ����������ظ����ϵζ�����2-3 ����
��ش���������:
��1���������У�����ȡ����ĵζ��ܼ��첿����������ȡҺ����ǰ������ʧ����ⶨ���____(����ƫ��������ƫС������������)��
��2���жϵ���ζ�·���������____________��
��3��������ʵ�����ݼ�¼��
�ζ����� | �������(mL) | NaOH��Һ�������(mL) | |
�ζ�ǰ | �ζ��� | ||
1 | 20.00 | 0.00 | 21.10 |
2 | 20.00 | 0.00 | 19.40 |
3 | 20.00 | 0.00 | 19.32 |
���ϱ����Կ�������1�εζ���¼��NaOH��Һ������Զ��ں����ε����������ܵ�ԭ����__________
A.NaOH ��Һ����ʱ��������в��ֱ���
B.��ƿ�ô���Һ��ϴ
C.����NaOH ��Һ���õ�ҩƷ�л���KOH����
D.�ζ�����ʱ�����Ӷ���
��4�������ϱ���¼���ݣ�ͨ������ɵã�������Ũ��Ϊ_____mol/L��
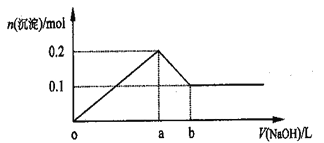
��5�������£���0.100mol/LNaOH��Һ�ֱ�ζ�20.00mL0.100mol/L������ʹ������ζ�������ͼ��ʾ������˵����ȷ����_________
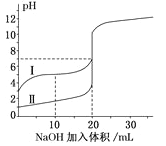
A.V(NaOH)=20mLʱ��c(Cl-)=c(CH3COO-)
B.I��ʾ���ǵζ����������
c.pH=7ʱ���ζ���������V(NaOH)С��20mL
D.V(NaOH)=10mLʱ��������Һ��:c(Na+)>c(CH3COO-)>c(H+)>c(OH-)
���𰸡� ƫС �����һ��NaOH��Һ���£���ƿ����Һ��ɫ����ɫ��ΪǮ��ɫ���Ұ�����ڲ���ɫ B 0.1936 C
����������1���ζ��ܼ��첿����������ȡҺ����ǰ������ʧ���൱����ȡ������������С�����ı�NaOH��Һ�����С���ⶨ���ƫС����ȷ�𰸣�ƫС��
��2����̪�������ɫ��������������Һ����Ϊ��ɫ�����Ե����һ��NaOH��Һ���£���ƿ����Һ��ɫ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ������ζ��յ㣻��ȷ�𰸣������һ��NaOH��Һ���£���ƿ����Һ��ɫ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
��3��NaOH��Һ����ʱ��������в��ֱ���Ϊ̼���ƣ�����NaOH��ҺŨ�ȱ�С�������һ�βⶨ���ƫ��������βⶨ���ҲӦƫ�ɽ��ƫС������NaOH��Һ�����ܱ�����A������ƿ�ô���Һ��ϴ���൱�����������������NaOH��Һ������B��ȷ���������NaOH��Һ���õ�ҩƷ�л���KOH���壬������Һ��Ũ��ƫС����һ����NaOH��Һ���ƫ�������εIJⶨ���Ҳ����ƫ���ƫС����������NaOH��Һ���õ�ҩƷ��û�л���KOH���壬C���ζ�����ʱ�����Ӷ��������NaOH��Һ���ƫС��D������ȷѡ��B��
��4����2��ƽ������NaOH��Һ���Ϊ19.36 mL�����빫ʽ���м�����c(HCl)=c(NaOH)��V(NaOH)/V(HCl)=0.2��19.36��10-3/20��10-3=0.1936 mol/L����ȷ����0.1936��
��5��0.100mol/L������ʹ��ᣬpH=1��pH>1����ͼ�����߱仯��֪I��ʾ���ǵζ�����
�����ߣ�B����V(NaOH)=20mLʱ�����ǡ����ȫ��Ӧ�������Ȼ��ƺʹ����ƣ��Ȼ��Ʋ�ˮ�������ԣ�������ˮ���Լ��ԣ�����c(Cl-)>c(CH3COO-)��A����V(NaOH)=20mLʱ���������ɴ�������Һ���Լ��ԣ��������ԣ�����͵��ʵ�������Ҳ���ǵζ���������V(NaOH)С��20mL�� C��ȷ��V(NaOH)=10mLʱ��������ҺΪ�����ƺʹ��ᣨ1:1���Ļ��Һ����Һ�����ԣ���������������ڴ��������ˮ������������c(CH3COO-)> c(Na+)��D������ȷѡ��D��
.

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�