��Ŀ����
����Ŀ��(1)���¶Ժ�������˶�״̬��������ȷ����_____(����)
A.�ܲ�����Խ��s�����Ƶİ뾶Խ��
B.��ͬһ�ܼ����˶��ĵ��ӣ����˶�״̬�϶���ͬ
C.���ӵ��˶����������ƣ�Χ��ԭ�Ӻ��ڹ̶��Ĺ���ϸ�����ת
D.�����͵ĵ���ֻ����s������˶��������ߵĵ���������f������˶�
(2)������һЩԭ�ӵ�2p�ܼ���3d�ܼ��е����Ų��������������ȷ����____(����)
A.![]() B.
B.![]() C.
C.![]() D.
D.![]()
(3)����˵����ȷ����_____(����)
A.���������ж����ڻ�ѧ��
B. �Ҽ��ͦм���ֻ�ܴ����ڹ��۷�����
C.ȫ���ɷǽ���Ԫ����ɵĻ������п϶����������Ӽ�
D.���������ֻ���ڦҼ�����6��C��H����1��C��C����Ϊ�Ҽ��������ڦм�
���𰸡� A C D
��������(1)A���ܲ�����Խ��sԭ�ӹ��������Խ�ߣ�����İ뾶Խ��ѡ��A��ȷ��B����ͬһ�ܼ����˶��ĵ��ӣ����˶�״̬��������ͬ����Ϊ��ͬһ���������ʱ�����������෴��ѡ��B����C�������˶�����Χ��ԭ�Ӻ��ڹ̶��Ĺ���ϸ�����ת��ֻ����ij��������ֵĸ��ʴ�Щ��ѡ��C����D�������ߵĵ���Ҳ������s������˶� ��7s����ϵĵ�������Ҳ�ܸߣ���4f�������ߣ�ѡ��D����ѡA��(2)A��ѡ����2p�������Ե�������������ͬ��ÿ��������ֻ���������������������෴��ѡ��A����B��������ܼ���ͬ�Ĺ����ֻ�б�������һ����ƽ�е�ռ�ݺ������ɵڶ������ӣ�ÿ��������ֻ���������������������෴����B����C��3d�ܼ��е����Ų����ϼ�����ܼ���ͬ�Ĺ����ֻ�б�������һ����ƽ�е�ռ�ݺ������ɵڶ������ӣ�ÿ��������ֻ���������������������෴��ѡ��C��ȷ��D��3d����е�2�������ӣ���������ͬ��ѡ��D����ѡC��(3)A����ԭ�ӷ��Ӳ����л�ѧ������He�ȣ�ѡ��A����B. ���ӻ�����Ҳ���ܴ��ڹ��ۼ�����Ҽ��ͦм����ܴ����������У�ѡ��B����C����β��ɷǽ���Ԫ�ع��ɣ�Ϊ���ӻ�������Ȼ�淋ȣ��������Ӽ���ѡ��C����D.���������ֻ���ڦҼ�����6��C��H����1��C��C����Ϊ�Ҽ��������ڦм���ѡ��D��ȷ����ѡD��

 ��ʦָ����ĩ��̾�ϵ�д�
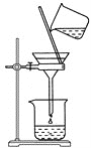
��ʦָ����ĩ��̾�ϵ�д�����Ŀ��ijѧ����0.2000mol/L�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ���������: ���ñ���Һ��ϴ�ζ���2-3 �Σ�ȡ��NaOH ��Һע���ʽ�ζ�������0���̶������������̶��õζ��ܲ�ʹ�ζ��ܼ������Һ����������Һ������0������0���̶������£������¶���������ȡ20.00mL����Һע���徻����ƿ�У�������3�η�̪��Һ�����ñ�Һ�ζ����յ������µζ���Һ����������ظ����ϵζ�����2-3 ����
��ش���������:
��1���������У�����ȡ����ĵζ��ܼ��첿����������ȡҺ����ǰ������ʧ����ⶨ���____(����ƫ��������ƫС������������)��
��2���жϵ���ζ�·���������____________��
��3��������ʵ�����ݼ�¼��
�ζ����� | �������(mL) | NaOH��Һ�������(mL) | |
�ζ�ǰ | �ζ��� | ||
1 | 20.00 | 0.00 | 21.10 |
2 | 20.00 | 0.00 | 19.40 |
3 | 20.00 | 0.00 | 19.32 |
���ϱ����Կ�������1�εζ���¼��NaOH��Һ������Զ��ں����ε����������ܵ�ԭ����__________
A.NaOH ��Һ����ʱ��������в��ֱ���
B.��ƿ�ô���Һ��ϴ
C.����NaOH ��Һ���õ�ҩƷ�л���KOH����
D.�ζ�����ʱ�����Ӷ���
��4�������ϱ���¼���ݣ�ͨ������ɵã�������Ũ��Ϊ_____mol/L��
��5�������£���0.100mol/LNaOH��Һ�ֱ�ζ�20.00mL0.100mol/L������ʹ������ζ�������ͼ��ʾ������˵����ȷ����_________

A.V(NaOH)=20mLʱ��c(Cl-)=c(CH3COO-)
B.I��ʾ���ǵζ����������
c.pH=7ʱ���ζ���������V(NaOH)С��20mL
D.V(NaOH)=10mLʱ��������Һ��:c(Na+)>c(CH3COO-)>c(H+)>c(OH-)