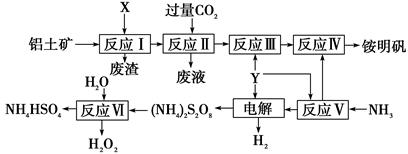
��Ŀ����
ij��ɫ��Һ����Na����Ag����Ba2����Al3����AlO2����MnO4����CO32����SO42����SiO32���е���
�������(������ˮ�ĵ���)��ȡ����Һ��������ʵ�飺
��.ȡ������Һ��������������ᣬ���������ɣ����õ���ɫ��Һ��
��.�ڢ�������Һ�м��������NH4HCO3��Һ�����������ɣ�ͬʱ������ɫ�����ף�
��.�ڢ�������Һ�м��������Ba(OH)2��Һ�����ȣ�Ҳ���������ɣ�ͬʱ������ɫ�����ҡ�
��ش��������⣺
(1)��ʵ����֪ԭ��Һ��һ�������е�������________��һ�����е�������________________��
(2)��ʵ����֪ԭ��Һ�л�һ�����е�������________�����ɼ����ӷ���ʽΪ________________��
(3)ʵ��������ɰ�ɫ�����ҵ����ӷ���ʽΪ________________��
(4)ԭ��Һ�л����ܴ��ڵ�������________����������ӵķ�����_________________��
(1)MnO4����SiO32����Ag����Ba2����Al3����CO32����Na��
(2)AlO2����3HCO3����Al3��=Al(OH)3����3CO2��
(3)2OH����NH4+��HCO3����Ba2�� BaCO3����NH3����2H2O
BaCO3����NH3����2H2O
(4)SO42����ȡ��ɫ�����������������Թ��У����������ϡ�����ϡ���ᣬ�����в��ְ�ɫ�������ܽ⣬��֤��ԭ��Һ����SO42��������ɫ����ȫ���ܽ⣬��֤��ԭ��Һ����SO42��
����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�ijУͬѧΪ̽��Br2��I2��Fe3����������ǿ��������������ʵ�顣
ʵ��٣�ȡ����KI��Һ���Թ��У��ȼ�����ˮ�����ټ���CCl4�����ã��۲쵽�²�Һ����Ϻ�ɫ��
ʵ��ڣ�ȡ����FeSO4��Һ���Թ��У��ȼ�����ˮ�����ټ����μ�����KSCN��Һ�����۲쵽��Һ�ʺ�ɫ��
(1)д�����ӷ���ʽ��
ʵ��٣�__________________________________________________________��
ʵ��ڣ�__________________________________________________________��
(2)����������ʵ�飬�����ʵ������Կ��Եó�����ȷ������________��
| A��Br2>I2 | B��Fe3��>Br2 |
| C��Br2>Fe3�� | D��I��>Br�� |
��FeCl3��Һ���ڵ�ˮ����KI��Һ����ϡH2SO4���ݵ�����Һ
__________________________________________________________________
__________________________________________________________________
ij�������Ƹ﹤ҵ������Cr�������������ù������£������ȡҺ�н���������Ҫ��
Cr3���������Fe3����Al3����Ca2����Mg2������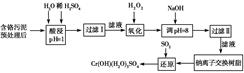
| �������� | Fe��OH��3 | Mg��OH��2 | Al��OH��3 | Cr��OH��3 |
| pH | 3.7 | 11.1 | 8 | 9����9��Һ�� |
��1�����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩΪ________________������дһ������
��2����pH��8��Ϊ�˳�ȥ________����Fe3����Al3����Ca2����Mg2������ͬ����
��3�������ӽ�����֬��ԭ��ΪMn����nNaR�D��MRn��nNa����������������������________��
��4������ƽ��Ӧ����ʽ��

 ��
������1 mol Cr��OH����H2O��5SO4����SO2�����ʵ���Ϊ________��



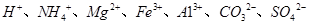 �е�ijЩ���ӣ��������Һ�м���B��Һʱ�������ɳ��������ʵ�����B��Һ����������仯��ͼ��ʾ���ɴ˿�֪������Һ�п϶����е����Ӽ���Ũ��֮��Ϊ ��
�е�ijЩ���ӣ��������Һ�м���B��Һʱ�������ɳ��������ʵ�����B��Һ����������仯��ͼ��ʾ���ɴ˿�֪������Һ�п϶����е����Ӽ���Ũ��֮��Ϊ ��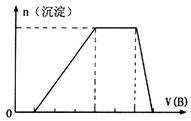
 ��ɫ����
��ɫ���� �������ܽ�
�������ܽ�