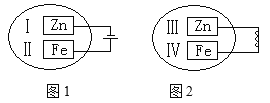��Ŀ����
����Ŀ����ͼΪ����ʱ��ͬpH����������Һ�к�������̬�ķֲ�������a��b��c�����Ӧ��pH�ֱ�Ϊ2.12��7.21��11.31����������ʾ�����������ʵ�������������˵����ȷ����
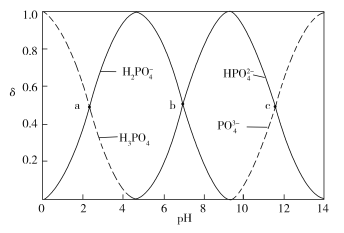
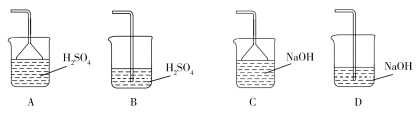
A.2 mol H3PO4��3 mol NaOH��Ӧ�����Һ������
B.NaOH��Һ�ζ�Na2HPO4��Һʱ�����÷�ָ̪ʾ�յ�
C.H3PO4�Ķ������볣����������Ϊ107
D.��Һ�г�OH�����⣬����������Ũ�����ʱ����Һ���������ԡ����Ի����
���𰸡�B
��������
2 mol H3PO4��3 mol NaOH��Ӧ�����ɵ����ʵ���Ũ�ȵ�NaH2PO4��Na2HPO4����ͼ�ɿ���b��![]() ��
��![]() Ũ����ȣ�pH=7.21���Լ��ԣ�Aѡ�����NaOH��Һ����Na2HPO4��Һʱ��������Ӧ
Ũ����ȣ�pH=7.21���Լ��ԣ�Aѡ�����NaOH��Һ����Na2HPO4��Һʱ��������Ӧ![]() +OH
+OH![]()
![]() +H2O����(
+H2O����(![]() )������(
)��С����(![]() )�����ڴ�pH�仯��Χ�ڣ����÷�ָ̪ʾ�յ㣬Bѡ����ȷ��H3PO4
)�����ڴ�pH�仯��Χ�ڣ����÷�ָ̪ʾ�յ㣬Bѡ����ȷ��H3PO4![]() H++
H++![]() ��
��![]()
![]() H++
H++![]() ��Ka2=
��Ka2=![]() ����c(
����c(![]() )=c(
)=c(![]() )ʱ��pH=7.21��c(H+)=107.21��������Ϊ108��Cѡ�������Һ��������Ũ�����ʱ��������c(
)ʱ��pH=7.21��c(H+)=107.21��������Ϊ108��Cѡ�������Һ��������Ũ�����ʱ��������c(![]() )=c(
)=c(![]() )����ʱ��ҺpH=7.21�Լ��ԣ���������c(
)����ʱ��ҺpH=7.21�Լ��ԣ���������c(![]() )=c(
)=c(![]() )����ʱpH=11.31���Լ��ԣ�Dѡ�����
)����ʱpH=11.31���Լ��ԣ�Dѡ�����

 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�����Ŀ����Դ�����ö�����̼�����ɼ�������������ŷţ��������»��ȼ�ϻ���Ҫ��ҵ��Ʒ��
��1����CO2��NH3Ϊԭ�Ͽɺϳɻ�������[CO��NH2��2]����֪��
��2NH3��g����CO2��g����NH2CO2NH4��s����H ����159.47 kJ��mol-1
��NH2CO2NH4��s����CO��NH2��2��s����H2O��g����H ��+116.49 kJ��mol-1
��H2O��l����H2O��g����H ��+88.0 kJ��mol-1
��д��NH3��CO2�ϳ����غ�Һ̬ˮ���Ȼ�ѧ����ʽ______________��
��2����֪��
��ѧ�� | Si��Cl | H��H | H��Cl | Si��Si |
����/kJ��mol��1 | 360 | 436 | 431 | 176 |
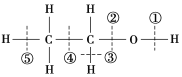
�ҹ辧����ÿ����ԭ�Ӻ�����4����ԭ���γ�4�����ۼ�����ҵ�����õĸߴ����ͨ������Ӧ����ȡ��SiCl4��g����2H2��g��![]() Si��s����4HCl��g�����÷�Ӧ����H��___ kJ��mol��1��
Si��s����4HCl��g�����÷�Ӧ����H��___ kJ��mol��1��
��3����һ�������£�������̼ת��Ϊ����ķ�Ӧ���£�CO2��g��+4H2��g��![]() CH4��g��+2H2O��g�� ��H��0��һ�ݻ�Ϊ2L�ĺ����ܱ������г���һ������CO2��H2����300��ʱ����������Ӧ���ﵽƽ��ʱ�����ʵ�Ũ�ȷֱ�ΪCO2��0.2mol��L-1��H2��0.8mol��L-1��CH4��0.8mol��L-1��H2O��1.6mol��L-1����ʼ����CO2��H2�����ʵ����ֱ�Ϊ_____��_____��CO2��ƽ��ת����Ϊ______��
CH4��g��+2H2O��g�� ��H��0��һ�ݻ�Ϊ2L�ĺ����ܱ������г���һ������CO2��H2����300��ʱ����������Ӧ���ﵽƽ��ʱ�����ʵ�Ũ�ȷֱ�ΪCO2��0.2mol��L-1��H2��0.8mol��L-1��CH4��0.8mol��L-1��H2O��1.6mol��L-1����ʼ����CO2��H2�����ʵ����ֱ�Ϊ_____��_____��CO2��ƽ��ת����Ϊ______��
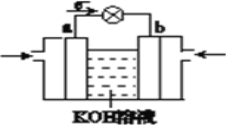
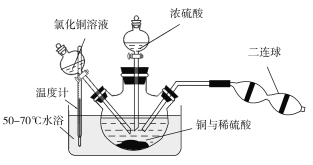
��4���۲���ͼ��ʾ������װ�ã�ͼ1װ����ͭ�缫�ϲ�����������ɫ���ݣ�ͼ2װ����ͭ�缫������������������缫�ϲ�����������ɫ���塣���������������Ʋ���������е�������Ҫ��ѧ����Ϊ
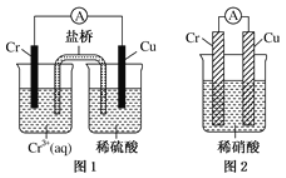
�� ____________________________________��
�� ____________________________________��