��Ŀ����
17����ҵ��ˮ�е�Cr2O72-��CrO42-����̬ϵͳ��ɺܴ�������л�ԭ��������һ�ֳ��õĴ���������������ͼ������˵����ȷ���ǣ�������CrO${\;}_{4}^{2-}$$��_{��ת��}^{H+}$Cr2O${\;}_{7}^{2-}$$��_{�ڻ�ԭ}^{Fe_{2}+}$Cr3+$��_{�۳���}^{������a}$����A��
| A�� | ����Aֻ��Cr��OH��3 | |
| B�� | �ڢٲ�ƽ����ϵ�У���������ϡ�������Һ��ɫ���ɫ����������CrO42-������ | |
| C�� | �ڢڲ��У���ԭ0.1mol Cr2O72-��Ҫ45.6gFeSO4 | |
| D�� | �ڢ۲�������a����ʹ��NaOH�ȼ������� |
���� A�����ݷ�Ӧ�ڶ�������ԭ Cr2O72-���������������۸��жϣ�
B����������ϡ�����������Ũ������ƽ�����ƣ���Һ���ɫ��������CrO42-�����ģ�
C������������ԭ��Ӧ�е�ʧ�����غ���㣻
D����Ϊ��Ӧ�ڶ�������ԭ Cr2O72-���������������۸������Եڢ۲�������a����ʹ��NaOH�ȼ�������������������������������
��� �⣺A����Ϊ��Ӧ�ڶ�������ԭ Cr2O72-���������������۸������Եڢ۲�������a����ʹ��NaOH�ȼ���������������������������������A����
B����������ϡ�����������Ũ������ƽ�����ƣ���Һ���ɫ��������CrO42-�����ģ���B����
C��Cr2O${\;}_{7}^{2-}$$��_{�ڻ�ԭ}^{Fe_{2}+}$Cr3+��1mol Cr2O72-��Ҫ6mol FeSO4���ʻ�ԭ0.1 mol Cr2O72-��Ҫ91.2 g FeSO4����C����
D����Ϊ��Ӧ�ڶ�������ԭ Cr2O72-���������������۸������Եڢ۲�������a����ʹ��NaOH�ȼ���������������������������������D��ȷ��
��ѡ��D��
���� ���⿼���˻�ѧƽ���Ӱ�����ء�ת�Ƶ������ļ����֪ʶ�㣬�ۺ��Ժ�ǿ���ѶȽϴ��ۺϿ�����ѧ���ķ�������������������

 ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д� һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д� �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д�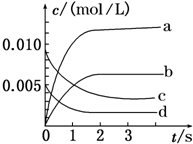 ��2L�ܱ������ڣ�800��ʱX��g����Y��g����Ӧ����Z��g������ϵ�У�n��X����ʱ��ı仯�����ʾ������֪��2X��g��+Y��g��?2Z��g�� ��
��2L�ܱ������ڣ�800��ʱX��g����Y��g����Ӧ����Z��g������ϵ�У�n��X����ʱ��ı仯�����ʾ������֪��2X��g��+Y��g��?2Z��g�� ��| ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
| n��X��/mol | 0.020 | 0.010 | 0.008 | 0.008 | 0.008 | 0.008 |
��2����ͼ�б�ʾZ�仯���ߵ���b����Y��ʾ��0��2s�ڸ÷�Ӧ��ƽ������v=1.5��10-3mol•L-1•s-1��
���������м������ϡH2SO4�����ˣ�
������Һ�м������KMnO4��Һ��������Һ��pHԼΪ3��
���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ��
��������MnSO4���Ϻ�ɫ��ʧ�����ˣ�
����Ũ�����ᾧ�����룬�õ���Ʒ��
��1��H2SO4�ܽ�A12O3�����ӷ���ʽ��Al2O3+6H+=2Al3++3H2O
��2��KMnO4����Fe2+�����ӷ���ʽ����������
1MnO4+5Fe2++8H+=1Mn2++5Fe3++4H2O
��3����֪��
�����������������pH
| Al��OH��3 | Fe��OH��2 | Fe��OH��3 | |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
���ݱ������ݽ��Ͳ�����Ŀ�ģ���ȥ��Ԫ��
��4����֪��һ�������£�MnO4-����Mn2+��Ӧ����MnO2��
�����ij����м���ŨHCI�����ȣ���˵�������д���MnO2��������Ũ������MnO2�������ɻ���ɫ����Cl2�����ж�MnO2�Ƿ���ڣ�
�ڢ��м���MnSO4��Ŀ���dz�ȥ������MnO4-��
| Ԫ�ط��ţ�Cs �������ƣ�� Ӣ�����ƣ�Cesium ԭ��������55 ���ԭ��������132.9 ��������Ų���2��8��18��18��8��1 |
| A�� | ����Ϊͬλ�� | |
| B�� | ����ԭ�Ӻ������������3 | |
| C�� | �����Ȼ���Ļ�ѧʽ�����Ա�ʾΪCsCl2 | |
| D�� | CsԪ��λ�����ڱ��������� |
| A�� | �ڴ�����Һ�м�������NaHSO4���壬����ĵ���ƽ�����ƣ�����Һ�е�c��H+����С | |
| B�� | ����ˮ���ȵ�100�棬���PH��7��˵�������¶ȿ�ʹˮ������ | |
| C�� | 0.2mol/L��������ˮ�������ϣ�������Һ��PH=1 | |
| D�� | PH=3��ϡ������PH=11�İ�ˮ�������ϣ�������Һ��PH=7 |
| A�� | 98g H2SO4 | B�� | 1NA��CO2 | C�� | 44.8LHCl | D�� | 6gH2 |