��Ŀ����
Fe��Al�����ֳ��õĽ����������ǰ�һ������������ɻ���
��1��ȡһ�������ĸû��������м���������NaOH��Һ��������������ڱ�״����Ϊ1.68L����Ӧ�����ӷ���ʽΪ______��������е�Al�����ʵ���Ϊ______��
��2����ȡ��ͬ�����ĸû��������м���������ϡ���ᣬ����ȫ���ܽ⣬�������������ڱ�״����ΪaL����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ______�����ú���ĸa����ѧʽ��ʾ��
��3����2�����õ���Һ�м������������������Һ���������ij������˳�������ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȣ���ԭ�������������������Ϊ______��
��4���ڱ�״���£�700LNH3ȫ���ܽ���1Lˮ�У�����Һ����������������ð�ˮ���ܶ�Ϊ0.85g?cm-3�������ʵ���Ũ��______��
��1��ȡһ�������ĸû��������м���������NaOH��Һ��������������ڱ�״����Ϊ1.68L����Ӧ�����ӷ���ʽΪ______��������е�Al�����ʵ���Ϊ______��
��2����ȡ��ͬ�����ĸû��������м���������ϡ���ᣬ����ȫ���ܽ⣬�������������ڱ�״����ΪaL����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ______�����ú���ĸa����ѧʽ��ʾ��
��3����2�����õ���Һ�м������������������Һ���������ij������˳�������ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȣ���ԭ�������������������Ϊ______��
��4���ڱ�״���£�700LNH3ȫ���ܽ���1Lˮ�У�����Һ����������������ð�ˮ���ܶ�Ϊ0.85g?cm-3�������ʵ���Ũ��______��
��1�������������Ʋ���Ӧ����������������Һ��Ӧ����ƫ�����ƺ����������ӷ�Ӧ����ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
���������ʵ���Ϊx��
2Al+2OH-+2H2O=2AlO2-+3H2��
2mol 67.2L
x 1.68L
=
�����x=0.5mol��
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����0.5mol��
��2���÷�Ӧ�е����غ㣬Al��Fe��ʧȥ����������H�õ�����������ת�Ƶ���Ϊ
��2����1-0��=
mol���ʴ�Ϊ��
mol��
��3����2�����õ���Һ�м������������������Һ���������ij������˳�������ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȣ�����������������������ԭ�������Al������������Al����������Ϊ
��100%=30%���ʴ�Ϊ��30%��
��4���ٶ�ˮΪ1L������Ϊ700L����
1Lˮ������Ϊ��1000mL��1g/mL=1000g��
���������ʵ���Ϊ
=31.25mol��������Ϊ31.25mol��17g/mol=531.25g��
������Һ������Ϊ1000g+531.25g=1531.25g����Һ�����Ϊ
��1801mL��1.8L��
�����ʵ���Ũ��Ϊ
=17.36mol/L��
�ʴ�Ϊ��17.36mol/L��
���������ʵ���Ϊx��
2Al+2OH-+2H2O=2AlO2-+3H2��
2mol 67.2L
x 1.68L
| 2 |
| x |
| 6.72L |
| 1.68L |
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����0.5mol��
��2���÷�Ӧ�е����غ㣬Al��Fe��ʧȥ����������H�õ�����������ת�Ƶ���Ϊ
| aL |
| 22.4L/mol |
| a |
| 11.2 |
| a |
| 11.2 |
��3����2�����õ���Һ�м������������������Һ���������ij������˳�������ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȣ�����������������������ԭ�������Al������������Al����������Ϊ
| 16��3 |
| 160 |
��4���ٶ�ˮΪ1L������Ϊ700L����
1Lˮ������Ϊ��1000mL��1g/mL=1000g��
���������ʵ���Ϊ
| 700L |
| 22.4L/mol |
������Һ������Ϊ1000g+531.25g=1531.25g����Һ�����Ϊ
| 1531.25g |
| 0.85g/mL |
�����ʵ���Ũ��Ϊ
| 31.25mol |
| 1.8L |
�ʴ�Ϊ��17.36mol/L��

��ϰ��ϵ�д�
�����Ŀ

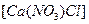 ��һ�ֻ��Σ���������
��һ�ֻ��Σ���������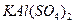 ��һ�ָ��Σ�����ʯ�������������ƣ�
��һ�ָ��Σ�����ʯ�������������ƣ� ��һ�����Ρ��������Ϊ
��һ�����Ρ��������Ϊ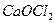 ���οɹ�����
���οɹ�����