��Ŀ����
����0.1 mol��L-1��AlCl3��Һ��0.1 mol��L-1��NaOH��Һ�����������ʵ�顣
(1)���Թ���ʢ������AlCl3��Һ10 mL������������������NaOH��Һ��
�ټ���10 mL NaOH��Һʱ��������___________������30 mL NaOH��Һʱ��������___________������35 mL NaOH��Һʱ��������___________��
�����ɳ����������ʱ����NaOH��Һ___________mL��
(2)��ʢ��10 mL NaOH��Һ���Թ��е���AlCl3��Һ��ͬʱ��ͣҡ���Թܣ����ֵ�������___________��������___________mL AlCl3��Һʱ��ʼ���ֳ�����������___________mL AlCl3��Һʹ���������ֵ��д���������̵����ӷ���ʽ
_______________________________��
(1)���Թ���ʢ������AlCl3��Һ10 mL������������������NaOH��Һ��
�ټ���10 mL NaOH��Һʱ��������___________������30 mL NaOH��Һʱ��������___________������35 mL NaOH��Һʱ��������___________��
�����ɳ����������ʱ����NaOH��Һ___________mL��
(2)��ʢ��10 mL NaOH��Һ���Թ��е���AlCl3��Һ��ͬʱ��ͣҡ���Թܣ����ֵ�������___________��������___________mL AlCl3��Һʱ��ʼ���ֳ�����������___________mL AlCl3��Һʹ���������ֵ��д���������̵����ӷ���ʽ
_______________________________��
(1)�������ɰ�ɫ��״��״ �������ﵽ���ֵ ��������ȥһ�� ��20
(2)��ʼ�������г��� 2.5 10/3
Al3++3OH-====Al(OH)3��
Al3++4OH-====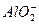 +2H2O
+2H2O
6H2O+Al3++3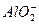 ====4Al(OH)3��
====4Al(OH)3��
(2)��ʼ�������г��� 2.5 10/3
Al3++3OH-====Al(OH)3��
Al3++4OH-====
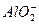 +2H2O
+2H2O6H2O+Al3++3
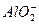 ====4Al(OH)3��
====4Al(OH)3����AlCl3��Һ����NaOH��Һ�������������ɰ�ɫ��״��������NaOH��Һ����ʱ���������ܽ⡣�����ӷ���ʽAl3++3OH-====Al(OH)3�������Ĺ�ϵ��֪��������30 mL NaOH��10 mLͬŨ��AlCl3��Һ�����������������ֵ������Al(OH)3+OH-="===" 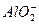 +2H2O���ӷ���ʽ��֪���ټ�10 mL��NaOH��Һ��������ȫ�ܽ⣬����ȥ����40 mL��NaOH����Al3+��ȫת��Ϊ
+2H2O���ӷ���ʽ��֪���ټ�10 mL��NaOH��Һ��������ȫ�ܽ⣬����ȥ����40 mL��NaOH����Al3+��ȫת��Ϊ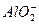 �����������ӷ���ʽAl3++4OH-====
�����������ӷ���ʽAl3++4OH-====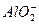 +2H2O������ڼ�35 mL NaOHʱ����������ȥһ�롣
+2H2O������ڼ�35 mL NaOHʱ����������ȥһ�롣
��NaOH��Һ�в��ϼ���AlCl3��Һʱ����ʼ������������Al3++3OH-====Al(OH)3���������ʱ��Һ������Al(OH)3����������Χ��Һ�ж���NaAlO2����Al3++4OH-====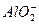 +2H2O��֪����0.1 mol��L-1��AlCl3��Һ�ﵽ0.1 mol��L-1 NaOH��Һ�����1/4ʱ��ԭNaOH��OH-�ľ�����Һ��Al3+ȫתΪ
+2H2O��֪����0.1 mol��L-1��AlCl3��Һ�ﵽ0.1 mol��L-1 NaOH��Һ�����1/4ʱ��ԭNaOH��OH-�ľ�����Һ��Al3+ȫתΪ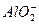 ������(2)����10/4 mL(2.5 mL)AlCl3ʱ��OH-�ڼ����������ꡣ�˺��ټ�AlCl3����������淴Ӧ��
������(2)����10/4 mL(2.5 mL)AlCl3ʱ��OH-�ڼ����������ꡣ�˺��ټ�AlCl3����������淴Ӧ��
6H2O+Al3++3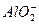 ====4Al(OH)3��
====4Al(OH)3��
��֪AlCl3��Һ�ճ���2.5 mL���г������ɡ�������ԭ���ӷ���ʽAl3++3OH-="===" Al(OH)3��ʱ��������࣬��10/3 mL AlCl3������������ֵ��
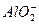 +2H2O���ӷ���ʽ��֪���ټ�10 mL��NaOH��Һ��������ȫ�ܽ⣬����ȥ����40 mL��NaOH����Al3+��ȫת��Ϊ
+2H2O���ӷ���ʽ��֪���ټ�10 mL��NaOH��Һ��������ȫ�ܽ⣬����ȥ����40 mL��NaOH����Al3+��ȫת��Ϊ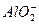 �����������ӷ���ʽAl3++4OH-====
�����������ӷ���ʽAl3++4OH-====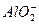 +2H2O������ڼ�35 mL NaOHʱ����������ȥһ�롣
+2H2O������ڼ�35 mL NaOHʱ����������ȥһ�롣��NaOH��Һ�в��ϼ���AlCl3��Һʱ����ʼ������������Al3++3OH-====Al(OH)3���������ʱ��Һ������Al(OH)3����������Χ��Һ�ж���NaAlO2����Al3++4OH-====
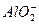 +2H2O��֪����0.1 mol��L-1��AlCl3��Һ�ﵽ0.1 mol��L-1 NaOH��Һ�����1/4ʱ��ԭNaOH��OH-�ľ�����Һ��Al3+ȫתΪ
+2H2O��֪����0.1 mol��L-1��AlCl3��Һ�ﵽ0.1 mol��L-1 NaOH��Һ�����1/4ʱ��ԭNaOH��OH-�ľ�����Һ��Al3+ȫתΪ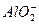 ������(2)����10/4 mL(2.5 mL)AlCl3ʱ��OH-�ڼ����������ꡣ�˺��ټ�AlCl3����������淴Ӧ��
������(2)����10/4 mL(2.5 mL)AlCl3ʱ��OH-�ڼ����������ꡣ�˺��ټ�AlCl3����������淴Ӧ��6H2O+Al3++3
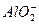 ====4Al(OH)3��
====4Al(OH)3����֪AlCl3��Һ�ճ���2.5 mL���г������ɡ�������ԭ���ӷ���ʽAl3++3OH-="===" Al(OH)3��ʱ��������࣬��10/3 mL AlCl3������������ֵ��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ