��Ŀ����
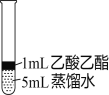
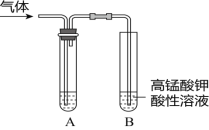
����Ŀ����ʵ�����Ƶ������г��������ʣ�Ӱ�������ʵļ��顣��ͼAΪ����װ�ã�BΪ���ʼ���װ�ã�������б���
��� | ���� | ��Ӧԭ�� | A���Լ� |
�� | ��ϩ | ��ˮ�Ҵ���Ũ���Ṳ�ȣ���Ӧ�Ļ�ѧ����ʽ��______ | ___ |
�� | ��ϩ | ��������NaOH���Ҵ���Һ���ȣ���Ӧ�Ļ�ѧ����ʽ��_____ | ___ |
�� | ��Ȳ | ���ʯ�еμӱ���ʳ��ˮ����Ӧ�Ļ�ѧ����ʽ��___ | ____ |
��Ϊ̽������������ˮ�������ijͬѧȡ��С��ͬ��3֧�Թܣ��ֱ����������Һ�����������ͬһˮԡ�м�����ͬʱ�䣬�۲쵽��������
�Թܱ�� | �� | �� | �� |
ʵ����� |
|
|
|
ʵ������ | ����䱡 | ������ʧ | ����������� |
��1���Թܢ��з�Ӧ�Ļ�ѧ����ʽ��__________��
��2������Թܢ�ʵ���������__________��
��3��ʵ�������__________��
���𰸡�CH3CH2OH ![]() CH2=CH2����H2O NaOH��Һ CH3CH2Br��NaOH
CH2=CH2����H2O NaOH��Һ CH3CH2Br��NaOH![]() CH2=CH2����NaBr+ H2O ˮ CaC2��2H2O��CH��CH����Ca��OH��2 CuSO4��Һ CH3COOC2H5��NaOH
CH2=CH2����NaBr+ H2O ˮ CaC2��2H2O��CH��CH����Ca��OH��2 CuSO4��Һ CH3COOC2H5��NaOH ![]() CH3COONa��C2H5OH �Ա�ʵ�飬̽������������ˮ������ ���������ڲ�ͬ�����µ�ˮ��̶ȣ����ԣ����ԣ�����
CH3COONa��C2H5OH �Ա�ʵ�飬̽������������ˮ������ ���������ڲ�ͬ�����µ�ˮ��̶ȣ����ԣ����ԣ�����
��������
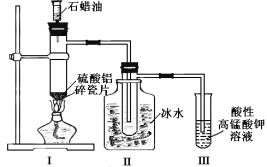
�����Ҵ�Ϊԭ������ϩ����Ҫ��Ũ��������������ˮ�����������¶���170�����ҡ�
��Ӧ�Ļ�ѧ����ʽΪCH3CH2OH ![]() CH2=CH2����H2O
CH2=CH2����H2O
����CH3CH2OH ![]() CH2=CH2����H2O��
CH2=CH2����H2O��
��Ϊ��ϩ�п��ܻ���Ũ���Ὣ�Ҵ�̼�������ɵ�SO2������Ӧʹ��NaOH��Һ���ӡ�
��Ϊ��NaOH��Һ��
������������NaOH���Ҵ���Һ��������ϩ��������Ӧ�Ļ�ѧ����ʽΪ
CH3CH2Br��NaOH![]() CH2=CH2����NaBr+ H2O��
CH2=CH2����NaBr+ H2O��
����CH3CH2Br��NaOH![]() CH2=CH2����NaBr+ H2O��
CH2=CH2����NaBr+ H2O��
��Ȼ����������ˮ��ĸ���Ӧ�������ﲻ������ϩ�����У���������ֻ��Ϊ�Ҵ������ܼ��ӷ����£�����ˮ�����Ҵ�����Ϊ��ˮ��
�����ʯ�еμӱ���ʳ��ˮ��������Ӧ�Ļ�ѧ����ʽΪCaC2��2H2O��CH��CH����Ca(OH)2��
����CaC2��2H2O��CH��CH����Ca(OH)2
��Ϊ��ʯ�г�����CaS���ʣ��������ɵ���Ȳ�����г�����H2S���壬��ʹ������ͭ��Һ��������Ϊ��CuSO4��Һ��
��1���Թܢ���Ϊ���������������������µ�ˮ�ⷴӦ����Ӧ��ȫ����Ӧ�Ļ�ѧ����ʽ��CH3COOC2H5��NaOH ![]() CH3COONa��C2H5OH��
CH3COONa��C2H5OH��
����CH3COOC2H5��NaOH ![]() CH3COONa��C2H5OH��
CH3COONa��C2H5OH��
��2���Թܢ���δ�Ӵ���������������ˮ�ⷴӦ������Ϊ�Ա�ʵ���õġ�
��Ϊ���Ա�ʵ�飬̽������������ˮ��������
��3�������������֪�����������ڼ�����������ȫˮ�⣻��ʵ������ķ�����֪�������������²���ˮ�⣻��ˮ��Һ�У���������ˮ��̶Ⱥ�С�����ԣ�ʵ����������������ڲ�ͬ�����µ�ˮ��̶ȣ����ԣ����ԣ�����
��Ϊ�����������ڲ�ͬ�����µ�ˮ��̶ȣ����ԣ����ԣ����ԡ�

 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д� ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�����Ŀ����50mL![]() �����50mL
�����50mL![]() NaOH��Һ�кͷ�Ӧ���������ų������������к��ȡ�
NaOH��Һ�кͷ�Ӧ���������ų������������к��ȡ�
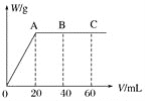
(1)�ձ���������ĭ���ϵ�������______��
(2)���ձ���������Ӳֽ�壬��õ��к�����ֵ______![]() ����ƫ��������ƫС��������Ӱ����
����ƫ��������ƫС��������Ӱ����![]()
(3)��ͼ��ʾ������A��������______��

(4)�ظ�����ʵ�飬��¼��ʵ���������£�
ʵ����Ʒ | ��Һ�¶� | |||
|
| |||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
��֪��![]() ����Ӧ����Һ�ı�����cΪ
����Ӧ����Һ�ı�����cΪ![]() �������ʵ��ܶȾ�Ϊ
�������ʵ��ܶȾ�Ϊ![]() ������
������![]() ______
______![]()
(5)ʵ���и���80mL![]() �����100mL
�����100mL![]() NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������______
NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������______![]() ������ȡ����������
������ȡ����������![]() �������к���______
�������к���______![]() ������ȡ����������
������ȡ����������![]() ��
��