��Ŀ����
����Ŀ���ش��������⣺
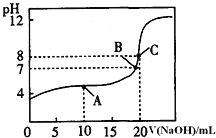
��1������ȥ�����������������(̼�ظ�)������Ũ�����У�10���Ӻ���������ͭ��Һ�У�Ƭ�̺�ȡ���۲죬�������������Ա仯����ԭ����__________________________��
��2������ȡ��ȥ�����������������(̼�ظ�)6��0g����15��0mlŨ�����У����ȣ����Ӧ��õ���ҺX���ռ�������Y��
����ͬѧ��ΪX�г�Fe3+����ܺ���Fe2+��д������Fe2+���п��ܵ����ӷ���ʽ��______________��
����ͬѧȡ336mL(��״��)����Yͨ��������ˮ�У�������Ӧ�Ļ�ѧ����ʽΪ��______________��
Ȼ���������BaCl2��Һ�����ʵ�������ø������2��33g�����ڴ���֪����Y��SO2���������Ϊ___________��
��3����100mLˮ��Ͷ��Na��Al��16�ˣ���ַ�Ӧ��ʣ�����1g������ų�H2�����Ϊ________��(��״����)
��4������0.3mol NaAlO2����Һ�еμ�1mol/L HCl��������7.8�˳���ʱ��������������Ϊ________mL
��5����һ������Fe��Fe2O3��CuO�����Ͷ��120 ml 2.2 mol/L��������Һ�У���ַ�Ӧ������896 mL��״���µ����壬�ò�����1.28 g�����˺�����Һ�м���2 mol/L��NaOH��Һ������40 mLʱ��ʼ���ֳ���������Һ��FeSO4�����ʵ���Ũ��Ϊ(����Һ���Ϊ120 ml)_________mol/L
���𰸡������汻�ۻ� Fe+2H+��Fe2++H2����Fe+ 2Fe3+��3Fe2+ SO2+Cl2+2H2O��2HCl+H2SO4 66��7% 13.44 100��900 1.87mol��L��1
��������
��1������(̼�ظ�)������Ũ�����У�Ũ�����н�ǿ����������ʹ�����ۻ���ֹ��Ӧ��һ�����У��ʴ�Ϊ�����汻�ۻ���
��2�����ڼ��������£�Ũ������Fe��Ӧ����Fe3+������ҺŨ�ȼ�Сʱ�����������Լ���������Fe2+����Ӧ�����ӷ���ʽΪFe+2H+=Fe2++H2������������ȫ���ģ���������ʱ�����ᷢ��Fe+2Fe3+=3Fe2+���ʴ�ΪFe+2H+=Fe2++H2���� Fe+2Fe3+=3Fe2+��
��SO2���л�ԭ�ԣ�ͨ��������ˮ�У�����SO2+Br2+2H2O=2HBr+H2SO4��n(�������)=![]() =0.015mol���������غ㣺n(SO2)=n(BaSO4)=
=0.015mol���������غ㣺n(SO2)=n(BaSO4)=![]() =0.01mol����SO2�����������
=0.01mol����SO2�����������![]() ��100%=66.7%���ʴ�ΪSO2+Br2+2H2O=2HBr+H2SO4��66.7%��
��100%=66.7%���ʴ�ΪSO2+Br2+2H2O=2HBr+H2SO4��66.7%��
��3��Al��ˮ����Ӧ������ʣ�����Ӧ����Al����Ӧ����ʽΪ2Na+2H2O=2NaOH+H2����2Al+2NaOH+2H2O=2NaAlO2+3H2��������Ӧ��ӣ�Al+Na+2H2O=2NaAlO2+2H2��������뷴Ӧ��Na��Al������֮��Ϊ23��27��Na��Al��������Ϊ15g������n(Na)=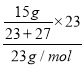 =0.3mol��n(Al)=0.3mol��n(H2)=2n(Na)=0.03mol��V(H2)=0.03mol��22.4L/mol=13.44L���ʴ�Ϊ13.44��
=0.3mol��n(Al)=0.3mol��n(H2)=2n(Na)=0.03mol��V(H2)=0.03mol��22.4L/mol=13.44L���ʴ�Ϊ13.44��
��4��n(Al(OH)3)=![]() =0.1mol��0.3mol��һ������ǣ���Ԫ�ش�����ƫ�����ƺ����������У���ʱ����������ֻ�ܳ���һ����AlO2-������NaAlO2+HCl+H2O=Al(OH)3��+NaCl�ã�n(HCl)=n(Al(OH)3)=0.1mol�����������=
=0.1mol��0.3mol��һ������ǣ���Ԫ�ش�����ƫ�����ƺ����������У���ʱ����������ֻ�ܳ���һ����AlO2-������NaAlO2+HCl+H2O=Al(OH)3��+NaCl�ã�n(HCl)=n(Al(OH)3)=0.1mol�����������=![]() =100mL��
=100mL��
��һ���������Ԫ�ش������Ȼ��������������У���ʱ����϶࣬�Ȱ�AlO2-ȫ�����������ֽ����ֳ����ܽ⣬��������������Ҫn(HCl)=n(NaAlO2)=n(Al(OH)3)=0.3mol���ܽ�Al(OH)3�����Ȼ�����Ҫ�����n(HCl)=3n(AlCl3)=3��(0.3-0.1)mol=0.6mol��������Ҫ�������=![]() =900mL���ʴ�Ϊ100��900��
=900mL���ʴ�Ϊ100��900��
��5�����������ϡ���ᷴӦ���й���ʣ�࣬˵����Һ�в����������ӣ�����Һ�м������2mol/L��NaOH��Һ������40mLʱ��ʼ���ֳ�����˵��������ʣ�࣬���Ⱥ�ͭ���Ӻ�������ӷ����û���Ӧ��������Һ�е����������ᡢ����������n(H2SO4)=2.2mol/L��0.12L=0.264mol��
���������Ʒ�Ӧ����������ʵ���=![]() n(NaOH)=
n(NaOH)=![]() ��2mol/L��0.04L=0.04mol��
��2mol/L��0.04L=0.04mol��
ʣ�����������ʵ���=0.264mol-0.04mol=0.224mol��
ʣ����������巴Ӧ����������������������������������Ĺ�ϵʽ�����������������ʵ���Ũ��=![]() =1.87mol/L���ʴ�Ϊ1.87��
=1.87mol/L���ʴ�Ϊ1.87��

 �Ķ��쳵ϵ�д�
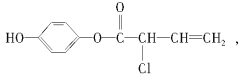
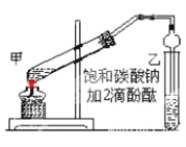
�Ķ��쳵ϵ�д�����Ŀ����ʵ�����Ƶ������г��������ʣ�Ӱ�������ʵļ��顣��ͼAΪ����װ�ã�BΪ���ʼ���װ�ã�������б���
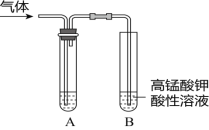
��� | ���� | ��Ӧԭ�� | A���Լ� |
�� | ��ϩ | ��ˮ�Ҵ���Ũ���Ṳ�ȣ���Ӧ�Ļ�ѧ����ʽ��______ | ___ |
�� | ��ϩ | ��������NaOH���Ҵ���Һ���ȣ���Ӧ�Ļ�ѧ����ʽ��_____ | ___ |
�� | ��Ȳ | ���ʯ�еμӱ���ʳ��ˮ����Ӧ�Ļ�ѧ����ʽ��___ | ____ |
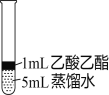
��Ϊ̽������������ˮ�������ijͬѧȡ��С��ͬ��3֧�Թܣ��ֱ����������Һ�����������ͬһˮԡ�м�����ͬʱ�䣬�۲쵽��������
�Թܱ�� | �� | �� | �� |
ʵ����� |
|
|
|
ʵ������ | ����䱡 | ������ʧ | ����������� |
��1���Թܢ��з�Ӧ�Ļ�ѧ����ʽ��__________��
��2������Թܢ�ʵ���������__________��
��3��ʵ�������__________��