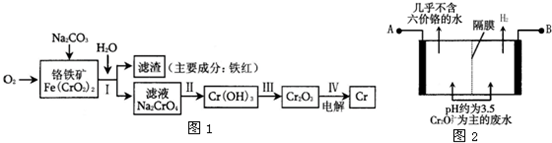
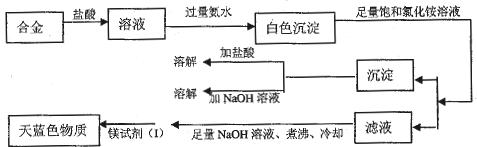
��Ŀ����
��10�֣�ʯ����Դ����������Լ�й���չ�γ���ҵ����������Լ�γ������ͥ����Ҫ���أ���2004��5��23�յġ������������������й��������ƹ㡰�����Ҵ����͡����Ҵ���ȫȼ������CO2��H2O��
��1��д���Ҵ���ȫȼ�յĻ�ѧ����ʽ______________________________________��
��2���Ҵ�ȼ��ʱ����������㣬���ܻ���CO���ɣ�������װ��ȷ֤�Ҵ�ȼ�ղ�����CO��CO2��H2O��Ӧ���Ҵ�ȼ�ղ�������ͨ����������������˳����װ�ñ�ţ���__________
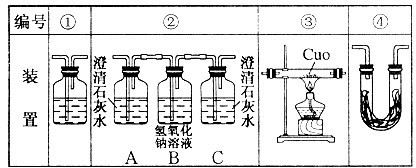
��3��ʵ��ʱ�ɹ۲쵽װ�â���Aƿ��ʯ��ˮ����ǣ�Cƿ��ʯ��ˮ������ǣ�Aƿ��Һ��������___________________________��Bƿ��Һ��������________________________��Cƿ��Һ��������______________________________________��
��4��װ�â۵������ǽ�CO������CO2��װ�â�����ʢ����_________________��Һ��������________________________________��
��5��װ�â�����ʢ�Ĺ���ҩƷ��________________��������ȷ֤������____________��
��1��д���Ҵ���ȫȼ�յĻ�ѧ����ʽ______________________________________��
��2���Ҵ�ȼ��ʱ����������㣬���ܻ���CO���ɣ�������װ��ȷ֤�Ҵ�ȼ�ղ�����CO��CO2��H2O��Ӧ���Ҵ�ȼ�ղ�������ͨ����������������˳����װ�ñ�ţ���__________

��3��ʵ��ʱ�ɹ۲쵽װ�â���Aƿ��ʯ��ˮ����ǣ�Cƿ��ʯ��ˮ������ǣ�Aƿ��Һ��������___________________________��Bƿ��Һ��������________________________��Cƿ��Һ��������______________________________________��
��4��װ�â۵������ǽ�CO������CO2��װ�â�����ʢ����_________________��Һ��������________________________________��
��5��װ�â�����ʢ�Ĺ���ҩƷ��________________��������ȷ֤������____________��
��10�֣���1��CH3CH2OH+3O2 2CO2 +3H2O ��2���ܢڢۢ٣�
2CO2 +3H2O ��2���ܢڢۢ٣�
��3����֤CO2���ڣ���ȥ��������е�CO2����֤��������е�CO2�Ƿ��ѱ�������
��4����CO������CO2������ʯ��ˮ[���������ơ�Ca(OH)2]��Һ��������CO��CuO��Ӧ�����ɵ�CO2���Ӷ�ȷ֤��CO���壮
��5������ˮ����ͭ����CuSO4��H2O����ˮ����
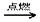 2CO2 +3H2O ��2���ܢڢۢ٣�
2CO2 +3H2O ��2���ܢڢۢ٣���3����֤CO2���ڣ���ȥ��������е�CO2����֤��������е�CO2�Ƿ��ѱ�������
��4����CO������CO2������ʯ��ˮ[���������ơ�Ca(OH)2]��Һ��������CO��CuO��Ӧ�����ɵ�CO2���Ӷ�ȷ֤��CO���壮
��5������ˮ����ͭ����CuSO4��H2O����ˮ����
��1���Ҵ���ȫȼ������CO2��ˮ������ʽΪCH3CH2OH+3O2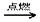 2CO2 +3H2O��
2CO2 +3H2O��
��2����֤COһ���ó��ȵ�����ͭ����֤ˮ����һ������ˮ����ͭ����֤CO2��һ���ó����ʯ��ˮ����������ͨ����Һһ�������ˮ����������������֤ˮ�����������ȷ��˳���Ǣܢڢۢ١�
��3��Aƿ��Һ����������֤CO2���ڣ�ƿ��Һ�������dz�ȥ��������е�CO2��Cƿ��Һ����������֤��������е�CO2�Ƿ��ѱ�������
��4��CO�ܱ�����ͭ��������CO2������װ�â�����ʢ���dz���ʯ��ˮ�������Ǽ�����CO��CuO��Ӧ�����ɵ�CO2���Ӷ�ȷ֤��CO���塣
��5��װ�â�����ʢ�Ĺ���ҩƷ����ˮ����ͭ��������ȷ֤������ˮ��
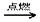 2CO2 +3H2O��
2CO2 +3H2O����2����֤COһ���ó��ȵ�����ͭ����֤ˮ����һ������ˮ����ͭ����֤CO2��һ���ó����ʯ��ˮ����������ͨ����Һһ�������ˮ����������������֤ˮ�����������ȷ��˳���Ǣܢڢۢ١�
��3��Aƿ��Һ����������֤CO2���ڣ�ƿ��Һ�������dz�ȥ��������е�CO2��Cƿ��Һ����������֤��������е�CO2�Ƿ��ѱ�������
��4��CO�ܱ�����ͭ��������CO2������װ�â�����ʢ���dz���ʯ��ˮ�������Ǽ�����CO��CuO��Ӧ�����ɵ�CO2���Ӷ�ȷ֤��CO���塣
��5��װ�â�����ʢ�Ĺ���ҩƷ����ˮ����ͭ��������ȷ֤������ˮ��

��ϰ��ϵ�д�
 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�
�����Ŀ