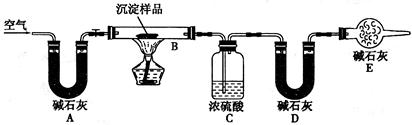
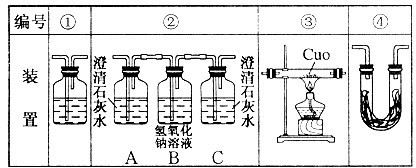
��Ŀ����
��8�֣�����̲�ʵ�������ո��л�ѧʵ��Ļ����������⻯ѧ��ѧ��ʵ��ԭ����ʵ�鷽����ʵ��˼·�����ѧ��ʵ�������Ļ���;����
��1��ʵ��1��ȡһ������ƣ��ڲ���Ƭ������ֽ���ɱ����ú�ͺ���С����ȥһ�˵���Ƥ���۲��Ƶ���ɫ�����ʵ���л����õ���һ�������� ��
��2��ʵ��2����һ��ʢ��ˮ��С�ձ�����뼸�η�̪��Һ��Ȼ���һС����Ͷ��С�ձ����ѷ�Ӧ���������Ӧ��������ո�����---�Ƶ��ܶȱ�ˮС�����ۡ�--- �����족---��Ӧ�������������ƣ�
��3��ʵ��3������֧�Թ���ֱ����5mL �����5mL NaOH��Һ���ڸ�����һС����Ƭ���۲�ʵ��������д������NaOH��Һ��Ӧ�����ӷ���ʽ�� ��
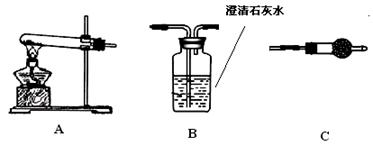
��4��ʵ��4����ˮ����ʢ��Na2O2������Թ��У������ô����ǵ�ľ�������Թܿڣ��������ɵ����塣��Ӧ�����Һ�м����̪��Һ�����Կ��� ��
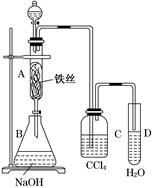
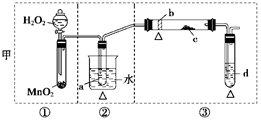
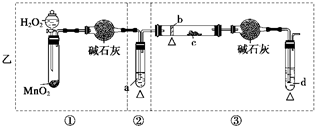
��5��ʵ��5���ڲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ��������������ԭ��Ӧ���������� ��
��6��ʵ��6�����Թ���ע���������Ʊ���FeSO4��Һ���ý�ͷ�ι���ȡNaOH��Һ�����ιܼ�˲����Թ�����Һ�ײ�����������NaOH��Һ�����Կ�����ʼʱ����һ�ְ�ɫ����״��������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ������������ɫ�仯��ԭ���ǣ��û�ѧ����ʽ��ʾ���� ��
��1��ʵ��1��ȡһ������ƣ��ڲ���Ƭ������ֽ���ɱ����ú�ͺ���С����ȥһ�˵���Ƥ���۲��Ƶ���ɫ�����ʵ���л����õ���һ�������� ��
��2��ʵ��2����һ��ʢ��ˮ��С�ձ�����뼸�η�̪��Һ��Ȼ���һС����Ͷ��С�ձ����ѷ�Ӧ���������Ӧ��������ո�����---�Ƶ��ܶȱ�ˮС�����ۡ�--- �����족---��Ӧ�������������ƣ�
��3��ʵ��3������֧�Թ���ֱ����5mL �����5mL NaOH��Һ���ڸ�����һС����Ƭ���۲�ʵ��������д������NaOH��Һ��Ӧ�����ӷ���ʽ�� ��
��4��ʵ��4����ˮ����ʢ��Na2O2������Թ��У������ô����ǵ�ľ�������Թܿڣ��������ɵ����塣��Ӧ�����Һ�м����̪��Һ�����Կ��� ��
��5��ʵ��5���ڲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ��������������ԭ��Ӧ���������� ��
��6��ʵ��6�����Թ���ע���������Ʊ���FeSO4��Һ���ý�ͷ�ι���ȡNaOH��Һ�����ιܼ�˲����Թ�����Һ�ײ�����������NaOH��Һ�����Կ�����ʼʱ����һ�ְ�ɫ����״��������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ������������ɫ�仯��ԭ���ǣ��û�ѧ����ʽ��ʾ���� ��
��1��ʵ��1������ ��2��ʵ��2�����ۡ������Ƶ��۵�ͣ���Ӧ����
��3��ʵ��3��2Al+2OH-+2H2O=2AlO2-+3H2�� ��4��ʵ��4����Һ�ȱ�����ɫ
��5��ʵ��5��ˮ���� ��6��ʵ��6��4Fe��OH��2+O2+2H2O=4Fe��OH��3
��3��ʵ��3��2Al+2OH-+2H2O=2AlO2-+3H2�� ��4��ʵ��4����Һ�ȱ�����ɫ
��5��ʵ��5��ˮ���� ��6��ʵ��6��4Fe��OH��2+O2+2H2O=4Fe��OH��3
��1�����ǿ�״���壬Ӧ�������Ӽ�ȡ�����ơ�
��2����Ѹ���ۻ���˵���Ƶ��۵�ͣ��ҷ�Ӧ���ȡ�
��3�����ܺ�����������Һ��Ӧ����������ƫ�����ƣ�����ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
��4��������������ˮ�����������ƣ���Һ�Լ��ԣ�������Һ��ɺ�ɫ��ͬʱ�������ƻ�����Ư���ԣ������Һ�ĺ�ɫ������ȥ��
��5�����������ܺ�ˮ������Ӧ�����������������������ڷ�Ӧ�����ǻ�ԭ����ˮ��������������
��6���������������л�ԭ�ԣ����ױ�����������������������ʽΪ4Fe(OH)2+O2+2H2O=4Fe(OH)3��
��2����Ѹ���ۻ���˵���Ƶ��۵�ͣ��ҷ�Ӧ���ȡ�
��3�����ܺ�����������Һ��Ӧ����������ƫ�����ƣ�����ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
��4��������������ˮ�����������ƣ���Һ�Լ��ԣ�������Һ��ɺ�ɫ��ͬʱ�������ƻ�����Ư���ԣ������Һ�ĺ�ɫ������ȥ��
��5�����������ܺ�ˮ������Ӧ�����������������������ڷ�Ӧ�����ǻ�ԭ����ˮ��������������
��6���������������л�ԭ�ԣ����ױ�����������������������ʽΪ4Fe(OH)2+O2+2H2O=4Fe(OH)3��

��ϰ��ϵ�д�
�����Ŀ