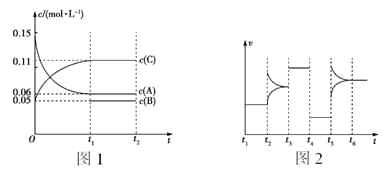��Ŀ����
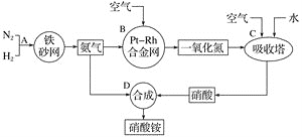
����Ŀ���������ƣ�Na2FeO4���Ǿ�����ɫ����ķ�ĩ����һ�ָ�Ч��ɫǿ�������������������ȶ��������ڷ�ˮ��������ˮ�Ĵ�����ʵ������ʯī������Ϊ�缫���Բ�ͬŨ�ȵ�NaOH��ҺΪ�������Һ������һ����ѹ����Ʊ��������ƣ����װ�ú�����������
c(NaOH) | �������� | �������� |
1 mol��L��1 | ������ɫ���� | ������ɫ���壬10min����Һ��ɫ�����Ա仯 |
10 mol��L��1 | ����������ɫ���� | ����������ɫ���壬3min����Һ��Ϊdz�Ϻ�ɫ��������� |
15 mol��L��1 | ����������ɫ���� | ����������ɫ���壬1min����Һ��Ϊdz�Ϻ�ɫ��������� |
����˵������ȷ����
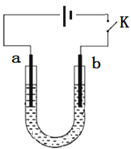
A. aΪ������bΪʯī
B. ������Ҫ������Ӧ��2H2O + 2e��=== H2��+ 2OH��
C. ��Ũ�ȵ�NaOH��Һ�������ڷ���Fe��6e��+ 8OH��=== FeO42��+ 4H2O
D. �Ʊ�Na2FeO4ʱ�����ñ���NaCl��Һ������Ч����������������
���𰸡�D
�������������������������Ϣ��֪������Ϊ������ʯīΪ������������ˮ����������ӷŵ�����������������������Һ��Ũ�Ⱥܴ�ʱ�������ϼ������������ӷŵ�����������������������ΪFeO42����A. aΪ������bΪʯī��A��ȷ��B. ������Ҫ�����ĵ缫��Ӧ��2H2O + 2e��=== H2��+ 2OH����B��ȷ��C. ��Ũ�ȵ�NaOH��Һ�������ڷ���Fe��6e��+ 8OH��=== FeO42��+ 4H2O��C��ȷ��D. �Ʊ�Na2FeO4ʱ�����ñ���NaCl��Һ�������������ӷŵ����������D����ȷ������ѡD��

����Ŀ������CH4������NO2����Ⱦ����Ӧԭ��Ϊ��CH4(g)+2NO2(g) N2(g) + CO2(g) +2H2O(g)����10L�ܱ������зֱ����0.50mol CH4 ��1.2mol NO2����ò�ͬ�¶��� n(CH4)��ʱ��仯���й�ʵ�����������ʾ������˵����ȷ����
��� | �¶�/K | ʱ��/min ���ʵ���/mol | 0 | 10 | 20 | 40 | 50 |
�� | T1 | n(CH4) | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
�� | T2 | n(CH4) | 0.50 | 0.30 | 0.18 | M | 0.15 |
A.��ʵ�����ݿ�֪�¶� T 1��T2
B.������ 0 ~20 min �ڣ�NO2 ��������Ϊ0.0125 molL-1min-1
C.40 min ʱ�������� M ��Ӧ������Ϊ 0.18
D.�÷�Ӧֻ���ڸ����²����Է�����
����Ŀ�����к͵ζ����ⶨ�ռ�Ĵ��ȣ����ռ��к����������������õĿ��������ʣ��Ը���ʵ��ش�
��1��ȷ��ȡ4.0g�ռ���Ʒ��
��2������Ʒ���250mL����Һ��
��3����____________�����������ƣ���ȡ25.00mL����Һ����ƿ�У����μӼ��μ�����ָʾ����
��4����0.2010 mol��L����������ζ������ռ���Һ���ζ�ʱ������ע��____________��ֱ���ζ��յ㡣�ﵽ�յ�ľ��������ǣ�____________��
��5��������ʵ��ζ����������±���
�ζ����� | ����Һ�����mL�� | �����������mL�� | |
�ζ�ǰ������mL�� | �ζ��������mL�� | ||
��һ�� | 25.00 | 0.50 | 20.40 |
�ڶ��� | 25.00 | 5.00 | 28.30 |
������ | 25.00 | 4.00 | 24.10 |
�������������ݣ������ռ�Ĵ��ȣ�____________
��6�����в����У��ᵼ������õ��ռ�Ĵ���ƫ�����________��
a���ζ��յ�ʱ�����ӿ̶�
b��û�����������Һ��ϴ��Ӧ�ĵζ���
c����ƿ��������������ˮ
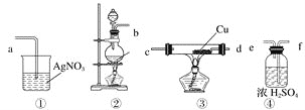
����Ŀ����ѧ��һ����ʵ��Ϊ������ѧ�ƣ�ʵ��̽���ܼ���ѧ��ѧϰ��ѧ����Ȥ��ij��ѧ��ȤС�������ͼʵ��װ�ã��г��豸���ԣ��Ʊ�������̽����������±��Ԫ�ص����ʡ��ش��������⣺
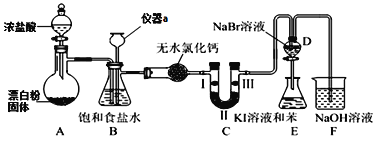
(1)����a��������______________��
(2)Aװ���з����Ļ�ѧ��Ӧ����ʽΪ_________________________________������Ư�ۻ���KClO3����Ӧ��ÿ����21.3g Cl2ʱת�Ƶĵ�����ĿΪ____NA��
(3)װ��B�����ڼ��ʵ�������C���Ƿ��������C�������˶�������B�пɹ۲쵽__________��
(4)װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ���ʱC�Т������������οɷ���____����ѡ��a��b��c����
ѡ�� | �� | �� | �� |
a | �������ɫ���� | Ũ���� | ʪ�����ɫ���� |
b | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |
c | ʪ�����ɫ���� | ��ʯ�� | �������ɫ���� |
(5)���װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ�����D�л���ͨ����������ʱ���ɹ۲쵽��ɫ��Һ��Ϊ����ɫ��˵���ȵķǽ����Դ����壬��������D��������Һ����E�У���E���۲쵽��������_______________________________��������_____����������������������˵����ķǽ�����ǿ�ڵ⣬ԭ����_____________________��