��Ŀ����
1Lij�����Һ�����ܺ��е��������±���
| ���ܴ������е������� | H+��Ag+��Mg2+��Al3+��NH4+��Fe3+ |
| ���ܴ������е������� | Cl-��Br-��I-��CO32-��Al(OH)4-(��AlO2-) |

��1������Һ��һ�������ڵ��������� ��
��2������Һ��һ�������ڵ��������� ��
��3��m��n��Ӧ�ĵ����ӷ���ʽΪ ��
��4����Һ�к��е������Ӷ�Ӧ�����ʵ�����Ϊ ��
����⣬����Һ�л����д�����Cl-��Br-��I-��������Һ�м����������Ȼ�����Һ����д���йط�Ӧ�����ӷ���ʽ ��
��1��Ag+��Mg2+��Fe3+��
��2��CO32- �� Al(OH)4-����AlO2-��
��3��NH4++OH-=NH3. H2O
��4��n(H+): n(NH4+):n(Al3+)=2:3:1
��2Fe3++2I-= I2+2Fe2+
���������������1��������Ŀ����ͼ������NaOH��Һ���������ӣ����ɳ���Ȼ��������ȫ�ܽ⣬˵��ԭ��Һ����Al3+����������ȫ�ܽ⣬˵������Һ����Ag+��Mg2+��Fe3+��
��2��Al3+����CO32- �� Al(OH)4-��Ӧ����������������һ�������ڡ�
��3��m��n�γ������仯��ΪNaOH��NH4+��Ӧ�����ӷ���ʽΪ��NH4++OH-=NH3.H2O��
��4������ͼ�����߿�֪����H+��Ӧ���ĵ�NaOH��Һ���Ϊ2V0����n(H+)="2" V0c(NaOH)����Al3+��Ӧ����Al(OH)3��������NaOH��Һ3V0����n(Al3+)= V0c(NaOH)����NH4+��Ӧ���ĵ�NaOH��Һ�����Ϊ3V0����n(NH4+)="3" V0c(NaOH)������n(H+): n(NH4+):n(Al3+)="2" V0c(NaOH)��3 V0c(NaOH)��V0c(NaOH)=2:3:1��
��Fe3+�������Դ���I2������Fe3+����I?����ΪI2�����ӷ���ʽΪ��2Fe3++2I-= I2+2Fe2+��
���㣺���⿼��ͼ��ķ�������Һ�����ӵ��жϡ����ӷ���ʽ���жϼ���ؼ��㡣

ij��Һ�н����±������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�������ӵ����ʵ�����Ϊ1mol��
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
������ԭ��Һ�м���KSCN��Һ�������Ա仯��������ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䡣��������Һ�м���BaCl2��Һ���а�ɫ���������Իش��������⡣
��1��������ԭ��Һ�м�����������ᣬ�ټ���KSCN��Һ�������� ��
��2��ԭ��Һ�к��е��������� ��
��3����ԭ��Һ�м������������ᣬ������Ӧ�����ӷ���ʽΪ ��
��4����ԭ��Һ�м���������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����գ��������ù�����������ƽ��������Ϊ ��
������������ԭ��ӦҲ�����ȷ�Ӧ����( )
| A�����ȵ�̿�������̼��Ӧ | B������ϡ����ķ�Ӧ |
| C��������������ķ�ĩ���Ȼ�茶����� | D��������Ʒ����ķ�Ӧ |
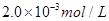 �������������Һ10.0mL��ǡ����ȫ��Ӧ���жϷ�Ӧ��ȫ��ʵ������Ϊ____________________________________,��üӵ�����Ʒ�е�Ԫ�صĺ���Ϊ______________________mg/Kg(�ú�w�Ĵ���ʽ��ʾ)��
�������������Һ10.0mL��ǡ����ȫ��Ӧ���жϷ�Ӧ��ȫ��ʵ������Ϊ____________________________________,��üӵ�����Ʒ�е�Ԫ�صĺ���Ϊ______________________mg/Kg(�ú�w�Ĵ���ʽ��ʾ)��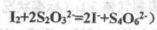 ��
��