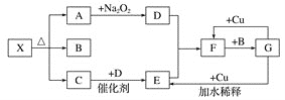
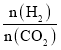��Ŀ����
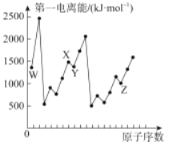
����Ŀ����������Ԫ��Qλ��ds�����������Ӱ����;������Ԫ��W��X��Y��Z��һ��������ԭ�������Ĺ�ϵ����ͼ��ʾ����ش���������(��Q��W��X��Y��Z����Ӧ��Ԫ�ط�������)��
��1����̬Yԭ�Ӻ����___���˶�״̬����ͬ�ĵ��ӡ�����n��ʾ�ܲ㣬����YԪ��ͬ���Ԫ�صĻ�̬ԭ�ӵļ۵����Ų�ʽΪ_________________��
��2��X��W��ɵ�һ�ֶ�Ԫ�����ﳣ�������ȼ�ϣ��û�������Xԭ�ӵ��ӻ���ʽΪ___________���û����ﳣ���³�Һ̬����е����Y2�е��ԭ��Ϊ___________��
��3��X2Y�����������������������ȵ�����ԭ����Ԥ��X2Y�Ŀռ乹��Ϊ______________��
��4��XW3���ڹµ��Ӷԣ����γ�[Q(XW3)4]2+���ӣ��������в�����_____________(�����)��
A.���Թ��ۼ� B.�Ǽ��Թ��ۼ� C.��λ�� D.���� E.����
��5��Q��X�γɵ�һ�ֶ�Ԫ����������������ṹ��ͼ��ʾ��
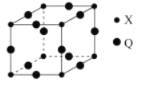
��Qԭ����Χ������������Qԭ�ӵ���ĿΪ_______��
�ڸö�Ԫ������Ļ�ѧʽΪ___________________��
��6����֪����Q����Ķѻ���ʽΪ�����������ܶѻ�������Q����ľ�����ԭ�ӵĿռ�������Ϊ_________________(�ú�����ʽ�ӱ�ʾ)��
���𰸡�8 ns2np4 sp3 N2H4��O2��Ϊ���Ӿ�������Է���������ͬ����N2H4����֮��������������е�ϸ� ֱ�� BE 8 Cu3N ![]()
��������
ͬ����Ԫ�ص�һ�����ܴ���������������ơ����� N ԭ��Ϊ 1s22s22p3�ﵽ�����ṹ����Խ��ȶ������Ե�һ�����ܻ�����������W��X��Y��Z��һ�����ܱ仯���ɿ�֪��WΪHԪ�ء�XΪNԪ�ء�YΪOԪ�ء�ZΪSԪ�أ���������Ԫ��Qλ��ds�����������Ӱ�������۵����Ų�ʽΪ3d104s1����QΪCu���ݴ˷�����
��1��YΪOԪ�أ���̬Oԭ����8�����ӣ�������8���˶�״̬����ͬ�ĵ��ӣ�OԪ�صĻ�̬ԭ�ӵļ۵����Ų�ʽΪ2s22p4������n��ʾ�ܲ㣬����YԪ��ͬ���Ԫ�صĻ�̬ԭ�ӵļ۵����Ų�ʽΪns2np4��
��2��X��W��ɵ�һ�ֶ�Ԫ������N2H4���������ȼ�ϣ�N2H4�����е�ԭ�ӵļ۲���Ӷ�=3+1=4������һ���µ��Ӷԣ�Nԭ�ӹ�����ӻ�������sp3��
N2H4��O2��Ϊ���Ӿ�������Է���������ͬ����N2H4����֮��������������е�ϸߣ�
��3��X2YΪN2O��������������������֪N2O��CO2��Ϊ�ȵ����壬�ȵ�����Ľṹ���ƣ���֪CO2Ϊֱ���εķ��ӣ�����N2O�Ŀռ乹��Ϊֱ���Σ�
��4��[Cu(NH3)4]2+��Cu2+��NH3֮��Ļ�ѧ��Ϊ��λ����N-HΪ���Թ��ۼ��������ڷǼ��Թ��ۼ���������
��ѡBE��
��5�����ɾ����ṹ��֪��Cu�ھ��������ϣ�Cuԭ����Χ������������Cuԭ���ھ���ͬ������ϣ���ĿΪ8��
�ڸ��ݾ����ṹ��֪��Cu�ھ��������ϣ��þ�����Cu�ĸ���Ϊ12![]() =3��N�ھ����Ķ����ϣ��þ�����N�ĸ���Ϊ8
=3��N�ھ����Ķ����ϣ��þ�����N�ĸ���Ϊ8![]() =1���ö�Ԫ������Ļ�ѧʽΪCu3N��
=1���ö�Ԫ������Ļ�ѧʽΪCu3N��
��6��Cu��������Ӷѻ���ʽΪ�����������ܶѻ����þ�����Cuԭ�Ӹ���=![]() ,�侧�����V= a3cm3�����ܶ�
,�侧�����V= a3cm3�����ܶ�![]() =
=![]() g/cm3=
g/cm3=![]() g/cm3��
g/cm3��
���ݾ����ṹ��֪��4r=![]() a�����a= 2
a�����a= 2![]() r��
r��
������������Ϊa3=(2![]() r)3��
r)3��
������4������ԭ�ӵ����Ϊ4![]() ��
��
���Դ˾�����ԭ�ӿռ�ռ������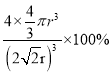 =
=![]() ��
��

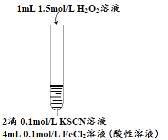
����Ŀ��ijС����Fe2+����ʵ���й۲쵽�쳣����Ϊ̽������ɫ��ȥ����ԭ��������ʵ�飺
��� | ʵ��I | ʵ��II | ʵ��III |
ʵ�鲽�� |
| ��ʵ��I��ɫ�����Һ�����ݷֱ����ʵ��
| Ϊ��һ��̽������ɫ��ȥ����ԭ���ֽ�������ʵ�� �� ��ȡ��Ӧ�����Һ���μ������BaCl2��Һ |
���� | ��Һ�ȱ�죬Ƭ�̺��ɫ��ȥ�����������ɣ�������ΪO2�� | ������������ ����Һ��� �۲�����ɫ���� | ����Һ��죬һ��ʱ�����ɫ�� ���ް�ɫ�������� |
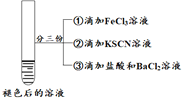
������������ʵ�飬������������ȷ����
A.�ڴ�ʵ��������H2O2����Fe2�������ʱ�����SCN�������ʿ�
B.ͨ��ʵ����Ƴ�ʵ����к�ɫ��ȥ��ԭ��������SCN��������
C.ͨ��ʵ����ʵ���Ա��Ƴ���ɫ��ȥֻ��H2O2���������й�
D.����������ʵ����к�ɫ��ȥ��ԭ���뻯ѧƽ���ƶ�ԭ����