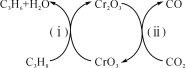
��Ŀ����
����Ŀ������Ԫ�������γɶ����������Ѫ��[K4Fe(CN)6]����³ʿ����Ѫ���ص�
��1����֪��3K4Fe(CN)6]=2KCN + Fe3C + 3C + (CN)2�� +2N2��
��(CN)2��������������������Ŀ��Ϊn(��)�sn(��)=____��
��(CN)2��һ���������⻯���Եõ��Ҷ�����H2NCH2CH2NH2�����Ҷ���������ˮ������Ϊ�Ǽ��Է����⣬�����ܵ�ԭ����____��
��2����³ʿ���Ļ�ѧʽΪFe4[Fe(CN)6]3��д��������ӻ�̬��������Ų�ʽ____��
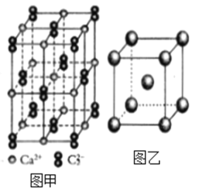
��3��Ѫ���أ���ͼ����Ѫ�쵰�ĺϳ�ԭ��֮һ��Ѫ��ɫ�е�Nԭ�ӵ��ӻ�����Ϊ____�����á�������ͼ�б��Fe2+����λ��_________��

���𰸡�3�s4 �Ҷ�����ˮ�γɷ��Ӽ���� [Ar]3d5 sp2��sp3 
��������
��1���ٵ���������������������1��������2����������(CN)2���ӵĽṹʽ��![]() ��(CN)2��������������������Ŀ��Ϊn(��)�sn(��)= 3�s4��
��(CN)2��������������������Ŀ��Ϊn(��)�sn(��)= 3�s4��
���Ҷ�����ˮ�γɷ��Ӽ�����������Ҷ���������ˮ��
����³ʿ���Ļ�ѧʽΪFe4[Fe(CN)6]3�����������Fe3+��Fe��Fe3+ʧȥ��4s����ϵ�2�����ӡ�3d����ϵ�1�����ӣ����Ի�̬Fe3+��������Ų�ʽ��[Ar]3d5��
��3�� ��Ȧ�����Nԭ���γɵ�̼˫���������γɵļ�Ϊ��λ������Nԭ�ӵ��ӻ���ʽ��sp2�������DZ���ĵ�ԭ���γ�3�������������γɹ��ۼ�����Nԭ�ӻ���1���µ��Ӷԣ������ӻ���ʽ��sp3���á�������ͼ�б��Fe2+����λ��Ϊ
��Ȧ�����Nԭ���γɵ�̼˫���������γɵļ�Ϊ��λ������Nԭ�ӵ��ӻ���ʽ��sp2�������DZ���ĵ�ԭ���γ�3�������������γɹ��ۼ�����Nԭ�ӻ���1���µ��Ӷԣ������ӻ���ʽ��sp3���á�������ͼ�б��Fe2+����λ��Ϊ ��
��

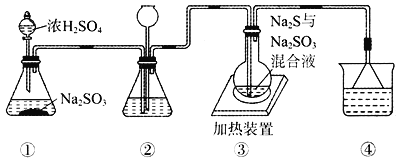
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�����Ŀ��������ѧ��ѧ����Ԫ��ԭ�ӽṹ�����������ʾ��
��� | Ԫ�� | �ṹ������ |
A | A�����������г������������������Ȼ����Է����������35.5 | |
B | Bԭ���������������ڲ����������1/5 | |
�� | C | C�dz������ʵ���ҪԪ�أ����ʳ����³���̬ |
�� | D | D���ʱ���Ϊ����Ϣ�����Ĵ��������dz��õİ뵼����� |
�� | E | ͨ������£�Eû�������ϼۣ�A��B��C��D��F������E�γɻ����� |
�� | F | F�����ڱ��п������ڢ�A�壬Ҳ����������ڢ�A�� |
(1)AԪ�������ڱ��е�λ��Ϊ____________________________________________��
(2)B��C�γɵĻ�����Ļ�ѧʽΪ________��������________(��������������������)�����
(3)��F��E�����γ�ԭ�Ӹ����ȷֱ�Ϊ2��1��1��1�����ֻ�����X��Y������X��Y��ˮ��Һ��ʵ�鷽����____________________
��F��C��ɵ����ֻ�����M��N�����ĵ������ֱ���X��Y��ȣ���M��ˮ��Һ��________�ԣ�N�ĽṹʽΪ________��
(4)C��E���ǽϻ��õķǽ���Ԫ�أ��û�ѧ����ʽ���������ֵ��ʵ�������ǿ��____��
(5)������ΪB��D�ĵ����õ������Ӻ�����Ȼ�����Һ�п����γ�ԭ��أ�����Ϊ�Ƿ���ԣ������ԣ���д�������ĵ缫����ʽ(����Ϊ���пɲ�д)___________________