ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΆ®≥Θ»ΥΟ«Α―≤πΩΣ1mol Ρ≥Μ·―ßΦϋΥυΈϋ ’ΒΡΡήΝΩΩ¥≥…ΗΟΜ·―ßΦϋΒΡΦϋΡήΓΘΦϋΡήΒΡ¥σ–ΓΩ…”Ο”ΎΙάΥψΜ·―ßΖ¥”ΠΒΡΖ¥”Π»»(ΓςH)ΓΘ
Μ·―ßΦϋ | ClΘ≠Cl | HΓΣH | HΓΣCl | NΓ‘N |
ΦϋΡή/kJΓΛmol | 243 | 436 | 431 | 946 |
(1)Α¥“Σ«σΆξ≥…ΧνΩ’
a.2HCl(g) ΘΫ H2(g)ΘΪCl2(g)ΘΜΓςH=______________________
b.N2 (g)+3H2(g)= 2NH3(g) ΠΛH=-92kJ/molΘ§‘ρNΓΣHΦϋΒΡΦϋΡή «__________kJΓΛmol
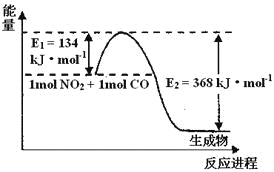
(2)1 mol NO2ΚΆ1mol COΖ¥”Π…ζ≥…CO2ΚΆNOΙΐ≥Χ÷–ΡήΝΩ±δΜ· Ψ“βΆΦΘ§«κ–¥≥ωNO2ΚΆCOΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ Ϋ__________________________________
(3) “―÷Σ‘Ύ≥ΘΈ¬≥Θ―Ιœ¬ΘΚ
ΔΌ 2CH3OH(l) ΘΪ 3O2(g) ΘΫ 2CO2(g) ΘΪ 4H2O(g) ΠΛH1
ΔΎ 2CO (g)+ O2(g) ΘΫ 2CO2(g) ΠΛH2
Δέ H2O(g) ΘΫ H2O(l) ΠΛH3
‘ρCH3OH(l)ΘΪO2(g)=CO(g)+ 2H2O(l)ΠΛH =__________(”ΟΚ§ΠΛH1ΓΔΠΛH2ΓΔΠΛH3ΒΡ ΫΉ”±μ Ψ)
(4)“―÷ΣΘΚ2Al (s)+ 3/2O2(g)==Al2O3(s) ΓςH=-1644.3 kJ mol-1
2Fe (s) +3/2O2(g)==Fe2O3(s) ΓςH=-815.88kJ mol-1
‘–¥≥ω¬ΝΖέ”κ―θΜ·ΧζΖέΡ©ΖΔ…ζ¬Ν»»Ζ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ Ϋ_______________________ΓΘ
ΓΨ¥πΑΗΓΩΓςH=+183kJ/mol 391 NO2(g) + CO(g) = CO2(g) + NO(g) ΓςH =-234kJΓΛmol-1 ![]() ΠΛH1-
ΠΛH1-![]() ΠΛH2+2ΠΛH3 2Al (s) + Fe2O3(s) === Al2O3(s) +2Fe (s) ΓςH= -828.42 kJ mol-1
ΠΛH2+2ΠΛH3 2Al (s) + Fe2O3(s) === Al2O3(s) +2Fe (s) ΓςH= -828.42 kJ mol-1
ΓΨΫβΈωΓΩ
(1)aΘ°ΗυΨίΨ…ΦϋΕœΝ―–η“ΣΈϋ ’ΡήΝΩΓΔ–¬Φϋ…ζ≥…–η“ΣΖ≈≥ω»»ΝΩΦΤΥψ≥ωΗΟΖ¥”ΠΒΡΖ¥”Π»»ΘΜ
bΘ°…ηN-HΦϋΒΡΦϋΡήΈΣxΘ§ΗυΨίΜ·―ßΦϋ”κΜ·―ßΖ¥”ΠΒΡΡήΝΩ±δΜ·ΒΡΙΊœΒΦΤΥψ≥ωΒΣ«βΦϋΒΡΦϋΡήΘΜ
(2)ΗυΨίΡήΝΩ±δΜ·ΆΦΘ§”Ο…ζ≥…ΈοΒΡΡήΝΩΦθΖ¥”ΠΈοΒΡΡήΝΩΨΆ «ΗΟΖ¥”Π»»ΘΜ
(3)Α―“―÷ΣΖ¥”ΠΖΫ≥Χ ΫΆ®Ιΐ±δΜ·±δ≥…Υυ«σΖΫ≥Χ ΫΘ§≤Δ«“Ζ¥”Π»»Ϋχ––œύ”ΠΒΡ±δΜ·Θ§ΉνΚσΥυΒΟΡήΝΩΨΆ «Υυ«σΖ¥”ΠΒΡΖ¥”Π»»ΘΜ
(4)Α―“―÷ΣΖ¥”ΠΖΫ≥Χ ΫΆ®Ιΐ±δΜ·±δ≥…Υυ«σΖΫ≥Χ ΫΘ§≤Δ«“Ζ¥”Π»»Ϋχ––œύ”ΠΒΡ±δΜ·Θ§ΉνΚσΥυΒΟΡήΝΩΨΆ «Υυ«σΖ¥”ΠΒΡΖ¥”Π»»ΓΘ
(1)aΘ°Ζ¥”Π2HCl(g)=H2(g)+Cl2(g)÷–Θ§ΕœΝ―2molH-ClΦϋ–η“ΣΈϋ ’ΒΡ»»ΝΩΈΣΘΚ431kJ/molΓΝ2=862kJ/molΘ§…ζ≥…1molH-HΦϋΚΆ1nolCl-ClΖ≈≥ωΒΡ»»ΝΩΈΣΘΚ243kJ/mol+436kJ/mol=679kJ/molΘ§œ‘»ΜΗΟΖ¥”ΠΈΣΈϋ»»Ζ¥”ΠΘ§Έϋ ’ΒΡ»»ΝΩΈΣΘΚ862kJ/mol-679kJ/mol=183kJ/molΘ§‘ρΓςH=+183kJ/molΘΜ
bΘ°N2(g)+3H2(g)=2NH3(g)ΓςH=-92kJ/molΘ§ΗΟΖ¥”ΠΈΣΖ≈»»Ζ¥”ΠΘ§ΥΒΟςΨ…ΦϋΕœΝ―Έϋ ’ΒΡ»»ΝΩ¥σ”Ύ–¬Φϋ…ζ≥…Ζ≈≥ωΒΡ»»ΝΩΘ§…η…ηN-HΦϋΒΡΦϋΡήΈΣxΘ§‘ρΜ·―ßΦϋΕœΝ―ΚΆ–Έ≥…Ιΐ≥Χ÷–ΡήΝΩ±δΜ·ΈΣΘΚxΓΝ2ΓΝ3-(946kJ/mol+436kJ/molΓΝ3)=92kJ/molΘ§ΫβΒΟx=391kJ/molΘΜ
(2)“ρΖ¥”ΠΈοΒΡΈο÷ ΒΡΝΩΕΦΈΣ1molΘ§Υυ“‘ΤδΖ¥”ΠΈοΒΡΦΤΝΩ ΐΈΣ1Θ§‘ΌΗυΨί‘≠Ή” ΊΚψ≈δΤΫΖΫ≥Χ ΫΘΜΗυΨίΆΦœσ÷ΣΘ§ΓςH=-(E2-E1)=-(368-134)kJ/mol=-234 kJmol-1Θ§Ι »»Μ·―ßΖΫ≥Χ ΫΈΣNO2(g)+CO(g)=CO2(g)+NO(g)ΓςH=-234 kJmol-1ΘΜ
(3)ΔΌ2CH3OH(l)+3O2(g)=2CO2(g)+4H2O(g)ΓςH1Θ§ΔΎ2CO(g)+O2(g)=2CO2(g)ΓςH2Θ§ΔέH2O(g)=H2O(l)ΓςH3Θ§‘ρCH3OH(l)+O2(g)=CO(g)+2H2O”…![]() ΔΌ-
ΔΌ-![]() ΔΎ+2ΔέΒΟΒΫΘ§Υυ“‘ΓςH=
ΔΎ+2ΔέΒΟΒΫΘ§Υυ“‘ΓςH=![]() ΓςH1-
ΓςH1-![]() ΓςH2+2ΓςH3ΘΜ
ΓςH2+2ΓςH3ΘΜ
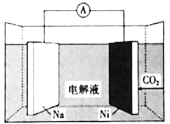
(4)¬Ν»»Ζ¥”ΠΒΡΖ¥”ΠΈο «¬ΝΖέ”κ―θΜ·ΧζΖέΡ©Θ§…ζ≥…Έο «ΧζΚΆ―θΜ·¬ΝΘΜ
2Al(s)+![]() O2(g)®TAl2O3(s)ΓςH=-1644.3kJmol-1 ΔΌΘ§2Fe(s)+
O2(g)®TAl2O3(s)ΓςH=-1644.3kJmol-1 ΔΌΘ§2Fe(s)+![]() O2(g)®TFe2O3(s)ΓςH=-815.88kJmol-1 ΔΎΘΜΖΫ≥Χ ΫΔΌ-ΔΎΒΟ2Al(s)+Fe2O3(s)
O2(g)®TFe2O3(s)ΓςH=-815.88kJmol-1 ΔΎΘΜΖΫ≥Χ ΫΔΌ-ΔΎΒΟ2Al(s)+Fe2O3(s)![]() Al2O3(s)+2Fe(s)ΘΜΥυ“‘ΓςH=ΓςH(ΔΌ)-ΓςH(ΔΎ)=-1644.3kJmol-1-(-815.88kJmol-1)=-828.42kJmol-1Θ§Ι »»Μ·―ßΖΫ≥Χ ΫΈΣΈΣΘΚ2Al(s)+Fe2O3(s)®TAl2O3(s)+2Fe(s)ΓςH=-828.42kJmol-1ΓΘ
Al2O3(s)+2Fe(s)ΘΜΥυ“‘ΓςH=ΓςH(ΔΌ)-ΓςH(ΔΎ)=-1644.3kJmol-1-(-815.88kJmol-1)=-828.42kJmol-1Θ§Ι »»Μ·―ßΖΫ≥Χ ΫΈΣΈΣΘΚ2Al(s)+Fe2O3(s)®TAl2O3(s)+2Fe(s)ΓςH=-828.42kJmol-1ΓΘ