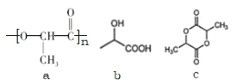
��Ŀ����
����Ŀ�������ǻ����л�����ԭ��֮һ���㷺����ũҩ��Ƥ�Ⱦ�ϡ�ҽҩ���ȹ�ҵ��
(1)��ҵ�����ü����������ϵת��ͼ��ͼ��

��Ӧ![]() ���ʱ�
���ʱ�![]() ________
________![]() ��
��
(2)ij��ѧС���о���ͬѹǿ�����Է�Ӧ![]() ��Ӱ�졣
��Ӱ�졣![]() �£���һ���ݻ��ɱ���ܱ������У�����һ������
�£���һ���ݻ��ɱ���ܱ������У�����һ������![]() ��
��![]() ����ò�ͬѹǿ�£�ƽ��ʱ�����������Ũ�����±���
����ò�ͬѹǿ�£�ƽ��ʱ�����������Ũ�����±���
������ | ��Ӧѹǿ | ����Ũ�� | ||
|
|
| ||
1 |
| 0.3 | 0.3 | 0.9 |
2 |
|
|
| 0.4 |
3 |
| 0.4 | 0.4 |
|
�Իش��������⣺
��ƽ��ʱ��ʵ��1������Ӧ����________(����>������<������=��)ʵ��3���淴Ӧ���ʡ�
����ʵ��1�����ݿɼ���![]() ʱ���÷�Ӧ��ƽ�ⳣ��
ʱ���÷�Ӧ��ƽ�ⳣ��![]() ________��
________��
��![]() ________
________![]() ��
��
(3)���о����ֲ��õ绹ԭ��Ҳ�ɽ�![]() ת��Ϊ�������ͬʱ�������
ת��Ϊ�������ͬʱ�������![]() ��ת��Ч�ʡ�����ԭ����ͼ��ʾ������������ȷ����________��
��ת��Ч�ʡ�����ԭ����ͼ��ʾ������������ȷ����________��
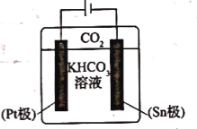
A.![]() ���ĵ缫��ӦʽΪ
���ĵ缫��ӦʽΪ![]()
B.��������![]() ��
��![]() ���ƶ�
���ƶ�
C.![]() ��������ԭ��Ӧ���������ݳ�
��������ԭ��Ӧ���������ݳ�
D.��������![]() Ũ����С
Ũ����С
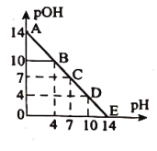
(4)�������;֮һ������������������Һ������������Һ�м���������ǿ��ʴ���Һ��![]() �仯�����ܱ�����Һ
�仯�����ܱ�����Һ![]() ����ȶ���(��֪����ĵ���ƽ�ⳣ��
����ȶ���(��֪����ĵ���ƽ�ⳣ��![]() )
)
���ֽ���Ũ�ȼ������������Һ��ϣ����![]() ��
��![]() ������Һ�������ӷ���ʽ��ʾ������ǿ�����
������Һ�������ӷ���ʽ��ʾ������ǿ�����![]() ������Һ�У�
������Һ�У�![]() �仯�����ԭ����________��
�仯�����ԭ����________��
������![]() ��Һ����
��Һ����![]() Ϊ4�Ļ�����Һ�������________
Ϊ4�Ļ�����Һ�������________![]() (�𰸱���һλС��)
(�𰸱���һλС��)![]() ��Һ��
��Һ��
���𰸡�+31.4 �� 10 0.2 AD HCOOH+OH-=HCOO-+H2O 642.9
��������
���ݸ�˹���ɣ������Ӧ�ȣ�������������ƽ��ʱʵ��3��ƽ��Ũ�ȴ���ʵ��1��ƽ��Ũ�ȣ��жϷ�Ӧ���ʴ�С����������ʵ��1���ݣ�����ƽ�ⳣ��Kֵ�������¶Ȳ��䣬Kֵ�������ʵ��2�е�a�����ݻ�����Һ��ԭ�����ͼ�������NaOH������кͣ�pH�仯�����ݻ�����Һ����ԭ���������NaOH�������
(1)��ͼʾ��֪��HCOOH(g)CO (g)+H2O(g)��H=+72.6kJ/mol����CO(g)+ ![]() O2(g)=CO2(g)��H=-283.0kJ/mol����H2(g)+
O2(g)=CO2(g)��H=-283.0kJ/mol����H2(g)+![]() O2(g)=H2O(g)��H=-241.8kJ/mol�����ø�˹���ɣ�����+��-�ۿɵ�HCOOH(g)CO2(g)+H2(g)���ʱ���H=��+72.6kJ/mol��+��-283.0kJ/mol��-��-241.8kJ/mol��=+31.4kJ/mol����Ϊ+31.4��
O2(g)=H2O(g)��H=-241.8kJ/mol�����ø�˹���ɣ�����+��-�ۿɵ�HCOOH(g)CO2(g)+H2(g)���ʱ���H=��+72.6kJ/mol��+��-283.0kJ/mol��-��-241.8kJ/mol��=+31.4kJ/mol����Ϊ+31.4��
(2)��ʵ��1��2��3���ں��º�ѹ�½��У��ɱ������ݿ�֪ʵ��3ƽ��Ũ�ȱ�ʵ��1�Ĵ���ѹǿ�ϴ�Ӧ���ʽϴ�ƽ��ʱ��ʵ��1������Ӧ����С��ʵ��3���淴Ӧ���ʣ���Ϊ����
����![]() ��H=-31.4 kJ/mol��֪��ƽ�ⳣ��K=
��H=-31.4 kJ/mol��֪��ƽ�ⳣ��K= ![]() ������ʵ��1�����ݼ�K=
������ʵ��1�����ݼ�K=![]() =10����Ϊ10��
=10����Ϊ10��
�۷�Ӧ����ͬ�¶��½��У�ƽ�ⳣ��K���䣬��K=10����֪K=![]() =
=![]() =10�����a=0.2����Ϊ0.2��
=10�����a=0.2����Ϊ0.2��
(3)A��Sn���Դ����������Sn��Ϊ������CO2�õ����ӷ�����ԭ��Ӧת��ΪHCOO-���缫��ӦΪCO2+2e-+HCO3-=HCOO-+CO32-����A��ȷ��
B�����������������������ƶ�����K+�������ƶ�������Sn���ƶ�����B����
C��Pt��Ϊ������ʧȥ���ӣ�����������Ӧ��2H2O-4e-=4H++O2������O2�ݳ�����C����
D�����������缫��ӦCO2+2e-+HCO3-=HCOO-+CO32-��֪����������HCO3-Ũ����С����D��ȷ��
��ΪAD��
(4)�ٽ�����ǿ�����HCOOH-HCOONa������Һ�У�HCOOH����NaOH��Ӧ������pH�仯�������ӷ���ʽΪHCOOH+OH-=HCOO-+H2O����Ϊ��HCOOH+OH-=HCOO-+H2O��
������100mL2molL-1HCOOH��Һ����pHΪ4��c(H+)=10-4mol/L�Ļ�����Һ������HCOOH![]() HCOO-+H+��֪��Ka=
HCOO-+H+��֪��Ka=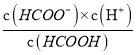 =
=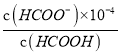 =1.8��10-4���ó�c(HCOO-)=1.8c(HCOOH)��ԭ��Һ��n(HCOOH)=0.2mol�������NaOH�����ʵ���Ϊx����ΪNaOH��HCOOH����HCOOH+NaOH=HCOONa+H2O��֪��n(NaOH)=n(HCOO-
=1.8��10-4���ó�c(HCOO-)=1.8c(HCOOH)��ԭ��Һ��n(HCOOH)=0.2mol�������NaOH�����ʵ���Ϊx����ΪNaOH��HCOOH����HCOOH+NaOH=HCOONa+H2O��֪��n(NaOH)=n(HCOO-![]() =
=![]() =0.6429L=642.9mL����Ϊ642.9��
=0.6429L=642.9mL����Ϊ642.9��

 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�