��Ŀ����
����Ŀ�����᳧�����ջ�����(FeS2)����ȡ���ᣬʵ�����������᳧����(��Ҫ�ɷ���Fe2O3������FeS��SiO2)�Ʊ��̷���
(1)SO2��O2��Ӧ��ȡSO3�ķ�Ӧԭ��Ϊ2SO2(g)��O2(g) ![]() 2SO3(g)����һ�ܱ�������һ��ʱ���ڴﵽƽ�⡣
2SO3(g)����һ�ܱ�������һ��ʱ���ڴﵽƽ�⡣
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪK��________��
�ڸ÷�Ӧ�ﵽƽ��״̬�ı�־��________��
A��v(SO2)��v(SO3)
B��������ƽ����Է�����������
C�����������������
D������ֵ������������
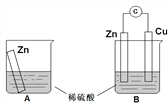
(2)ij���е�λ����ԭ���ԭ������SO2��O2���Ʊ����ᣬװ����ͼ���缫Ϊ��IJ��ϣ����������壬ͬʱҲ��ʹ������������Һ��ֽӴ���

�� B�缫�ĵ缫��Ӧʽ_______________________________________��
����Һ��H�����ƶ�������________����________��(��A��B��ʾ)��
(3)�ⶨ�̷���Ʒ�к�����ʵ�鲽�裺
a����ȡ5.7 g��Ʒ���ܽ⣬���250 mL��Һ
b����ȡ25 mL����Һ����ƿ��
c���������ữ��0.01 mol/L KMnO4��Һ�ζ����յ㣬����KMnO4��Һ���40 mL
������������ش��������⣺
�ٵζ�ʱ������Ӧ�����ӷ���ʽΪ(��ɲ���ƽ���ӷ�Ӧ����ʽ)��
________Fe2����________MnO![]() ��________===________Fe3����________Mn2����________
��________===________Fe3����________Mn2����________
�� �������ữ��KMnO4�ζ��յ�ı�־��
___________________________________
___________________________________��
�ۼ���������Ʒ��FeSO4��7H2O����������Ϊ________��
���𰸡�(1)��![]() ��BD
��BD
(2)��SO2��2e����2H2O===SO![]() ��4H����B A
��4H����B A
(3)��5��1��8��H����5��1��4��H2O
�ڵζ����һ������KMnO4ʱ��Һ�ʵ���ɫ��������ڲ���ɫ
��0.975��97.5%
��������(1)�ٸ��ݷ�Ӧ����ʽ2SO2��O2![]() 2SO3��ƽ�ⳣ��K��
2SO3��ƽ�ⳣ��K��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��
![]() ��
��
��A.v(SO2)��v(SO3)��û�и������淴Ӧ�����ж����淴Ӧ�����Ƿ���ȣ���A����
B����Ӧ����ʽ���߶������壬����Ļ�ѧ������֮�Ͳ���ȣ������ƽ����Է������Ǹ��仯������������ƽ����Է����������䣬˵�����淴Ӧ������ȣ��ﵽ��ƽ��״̬����B��ȷ��
C����Ӧ����ʽ���߶������壬���������ʼ�ղ��䣬���Ի�������������䣬�����ж��Ƿ�ﵽƽ��״̬����C����
D������ֵ�����������䣬���淴Ӧ������ȣ�˵���ﵽ��ƽ��״̬����D��ȷ��
��ѡ��BD��
(2)�ٸ�ԭ����У�������ʧ���ӱ����������Ը�����Ͷ�ŵ������Ƕ�������������ʧ���Ӻ�ˮ��Ӧ������������Ӻ������ӣ�������Ͷ�ŵ������������������������õ��Ӻ������ӷ�Ӧ����ˮ�����������ˮ�ij��ڷ���֪��B���Ǹ�����A��������������B���ϵĵ缫��ӦʽΪ��SO2��2e����2H2O===SO��4H����
�ʴ�Ϊ��SO2��2e����2H2O===SO![]() ��4H����
��4H����
��ԭ��طŵ�ʱ���������ɸ���B��������A���ʴ�Ϊ��B��A��
(3)���������ữ��0.01mol/L KMnO4��Һ�����Է�Ӧ����һ���������ӣ�����������ӻ��ϼ��ɣ�7��Ϊ��2��������5�ۣ����������ɣ�2��Ϊ��3��������1�ۣ����Ը����������ϵ��Ϊ1����������ϵ��Ϊ5�����ݵ���غ㡢�����غ���ƽ�����ӡ�ˮ����ƽ��ķ���ʽΪ��5Fe2����1MnO![]() ��8H��===5Fe3����1Mn2����4H2O��
��8H��===5Fe3����1Mn2����4H2O��
�ʴ�Ϊ��5��1��8��H����5��1��4��H2O��
�ڵ�����������������������ȫ��Ӧ���ٵ���һ�θ��������Һ����Һ��ʵ���ɫ���ݴ��жϵζ��յ㣬�ʴ�Ϊ���ζ����һ������KMnO4ʱ��Һ�ʵ���ɫ��������ڲ���ɫ��
��25 mL����Һ���ĵĸ�����ص����ʵ���Ϊ��0.01 mol/L��0.04 L��0.0004 mol��
5��7 g��Ʒ���250 mL��Һ���ĸ�����ص����ʵ���Ϊ0.0004 mol��![]() ��0.004 mol��
��0.004 mol��
���ݷ�Ӧ��5Fe2����1MnO![]() ��8H��===5Fe3����Mn2����4H2O���������������ʵ���Ϊ��0.004 mol��5��0.02 mol��
��8H��===5Fe3����Mn2����4H2O���������������ʵ���Ϊ��0.004 mol��5��0.02 mol��
������Ʒ�к��е�FeSO4��7H2O������Ϊ��278 g/mol��0.02 mol��5.56 g��
FeSO4��7H2O������������![]() ��100%��97.5%��
��100%��97.5%��
�ʴ�Ϊ��0.975��97.5%.

 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�