��Ŀ����
����Ŀ����ҵ������N2��H2����ʵ�ֺϳɰ����������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش������й����⣺
(1)��֪N2(g)+O2(g)=2NO(g) ��H=+180.5kJ��mol��1
N2(g)+3H2(g)![]() 2NH3(g) ��H=��92.4kJ.mol��1
2NH3(g) ��H=��92.4kJ.mol��1
2H2(g)+O2(g)=2H2O(g) ��H=��483.6kJ��mol��1
д����������������ȫ����һ��������ˮ�������Ȼ�ѧ����ʽΪ___________��
(2)��һ��������ܱ������У��������»�ѧ��Ӧ��N2(g)+3H2(g)![]() 2NH3(g)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
2NH3(g)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
t/K | 298 | 398 | 498 | ...... |
K/(mol/L)2 | 4.1��106 | K1 | K2 | ...... |
����������⣺
�ٱȽ�K1��K2�Ĵ�С��K1___________K2(����>������=������<��)��
����ͬ��ͬѹ���жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������___________(�����)��
A.2v(H2)(��)=3v(NH3)(��) B.2v(N2) (��)=v(H2) (��)
C.������ѹǿ���ֲ��� D.���������ܶȱ��ֲ���
(3)���Ṥҵ��β��NO�������Ʊ�NH4NO3���乤��ԭ����ͼ��
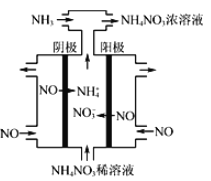
���������ĵ缫��ӦʽΪ___________��
�ڳ����£�1LpH=6��NH4NO3��Һ��c(NH3��H2O)+c(OH��)=___________mol��L��1��
(4)��ҵ���������صĻ�ѧ����ʽΪ��
2NH3(g)+CO2(g)![]() CO(NH2)2(s)+H2O(l)
CO(NH2)2(s)+H2O(l)
��T�棬���Ϊ4L���ܱ������У�ͨ��6molNH3��3 mol CO2����Ӧ�ﵽƽ��ʱ��c(NH3)=0.5mol��L��1��e(CO2)=0.25mol��L��1������ʱ����T���ƽ��ʱ������ѹǿ���䣬��������ɱ�������г���3molNH3�����ʱ��Ӧ��V��___________V��(����>����<������=��)���ٴ�ƽ���ƽ�ⳣ��Ϊ___________��
(5)��֪Ksp(AgCl)=1.8��10��10������50mL0.09mo1��L��1��AgNO3��Һ�м���50mL0.10mol��L��1�����ᣬ��Ϻ���Һ�е�Ag+��Ũ��Ϊ___________mol��L��1��
���𰸡�4NH3(g)��5O2(g)��4NO(g)��6H2O(g) ��H����905.0 kJ��mol��1 �� AD NO��5e����6H����NH4����H2O 10��6 �� 16 3.6��10��8
��������
(1)���ݸ�˹������д��������������ȫ����һ��������ˮ�������Ȼ�ѧ����ʽ��
(2) ��N2(g)+3H2(g)![]() 2NH3(g) ��H=��92.4kJ.mol��1����Ӧ���ȣ������¶�ƽ�������ƶ���
2NH3(g) ��H=��92.4kJ.mol��1����Ӧ���ȣ������¶�ƽ�������ƶ���
�ڸ���ƽ���־�жϣ�
(3) �������õ��ӷ�����ԭ��Ӧ���ڸ��������غ㣺NH4NO3��Һ��c(NH3��H2O)+c(OH��)= c(H+)��
(4)ƽ�ⳣ��K=![]() ������Q��K�Ĺ�ϵ�ж�V����V���Ĺ�ϵ��
������Q��K�Ĺ�ϵ�ж�V����V���Ĺ�ϵ��
(5)����Ksp(AgCl) =c(Cl��)c(Ag��)����Ag+��Ũ��
(1)��N2(g)+O2(g)=2NO(g) ��H=+180.5kJ��mol��1
��N2(g)+3H2(g)![]() 2NH3(g) ��H=��92.4kJ.mol��1
2NH3(g) ��H=��92.4kJ.mol��1
��2H2(g)+O2(g)=2H2O(g) ��H=��483.6kJ��mol��1
���ݸ�˹���ɢ١�2���ڡ�2���ۡ�3�� 4NH3(g)��5O2(g)��4NO(g)��6H2O(g) ��H����905.0 kJ��mol��1 ��
(2) ��N2(g)+3H2(g)![]() 2NH3(g) ��H=��92.4kJ.mol��1����Ӧ���ȣ������¶�ƽ�������ƶ���K��С������K1��K2��
2NH3(g) ��H=��92.4kJ.mol��1����Ӧ���ȣ������¶�ƽ�������ƶ���K��С������K1��K2��
��A.2v(H2)(��)=3v(NH3)(��) �����淴Ӧ���ʱȵ���ϵ���ȣ�һ���ﵽƽ��״̬����ѡA�� B.2v(N2) (��)=v(H2) (��) �����淴Ӧ���ʱȲ�����ϵ���ȣ�û�дﵽƽ��״̬���ʲ�ѡB��
C. N2(g)+3H2(g)![]() 2NH3(g) ��ͬ��ͬѹ�·�Ӧ��ѹǿ�Ǻ�����������ѹǿ���ֲ��䣬��һ���ﵽƽ��״̬���ʲ�ѡC��
2NH3(g) ��ͬ��ͬѹ�·�Ӧ��ѹǿ�Ǻ�����������ѹǿ���ֲ��䣬��һ���ﵽƽ��״̬���ʲ�ѡC��
D.����![]() ����ͬ��ͬѹ�·�Ӧ���������������䡢���������С�������ܶ��DZ��������������ܶȱ��ֲ��䣬һ��ƽ�⣬��ѡD��������������С��ѡAD��
����ͬ��ͬѹ�·�Ӧ���������������䡢���������С�������ܶ��DZ��������������ܶȱ��ֲ��䣬һ��ƽ�⣬��ѡD��������������С��ѡAD��
(3) ������������ԭ��Ӧ������ʾ��ͼ��NO�������õ��ӷ�����ԭ��Ӧ����NH4��,����������ӦʽΪNO��5e����6H����NH4����H2O��
��pH=6��NH4NO3��Һ�У�c(H+)=10��6 mol��L��1�����������غ㣺NH4NO3��Һ��c(NH3��H2O)+c(OH��)= c(H+)=10��6 mol��L��1��
(4)ƽ�ⳣ��K=![]() ��
��![]() 16��ͬ��ͬѹ�£�����ȵ������ʵ����ȣ���T���ƽ��ʱ������ѹǿ���䣬��������ɱ�������г���3molNH3���������ΪVL��
16��ͬ��ͬѹ�£�����ȵ������ʵ����ȣ���T���ƽ��ʱ������ѹǿ���䣬��������ɱ�������г���3molNH3���������ΪVL��![]() ��V=8L����ʱQ=
��V=8L����ʱQ=![]() ��
��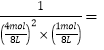 32��Q��K����Ӧ�������V����V����
32��Q��K����Ӧ�������V����V����
(5) ��50mL0.09mo1��L��1��AgNO3��Һ�м���50mL0.10mol��L��1�����������Ȼ���������������ʣ�࣬ʣ��c(Cl��)��(0.05L��0.10mol��L��1��0.05L��0.09mo1��L��1)��0.1L��0.005 mo1��L��1�� Ksp(AgCl) =c(Cl��)c(Ag��) ��1.8��10��10��c(Ag��) ��(1.8��10��10)��0.005 mo1��L��1��3.6��10��8 mol��L��1��