��Ŀ����
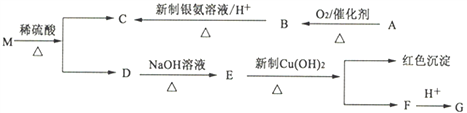
����Ŀ������ʽΪC9H8O2Br2���л�������M����һ�������¿ɷ�������һϵ�з�Ӧ��
��֪���������ǻ�ͬʱ����ͬһ̼ԭ���ϵĽṹ�Dz��ȶ��ģ�����������ˮ��Ӧ��

���л���A����Է�������Ϊ46���л���G��FeC13��Һ��ɫ���˴Ź���������4���塣
�ش��������⣺
��1��B��C�ķ�Ӧ������____________��
��2��M�Ľṹ��ʽΪ_________________________________________________��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
��A��B��________________________________________________________��
��D��E��________________________________________________________��
��E��F��________________________________________________________��
��4����G��Ϊͬ���칹�壬������������ȡ����������FeC13��Һ��ɫ��������_________�֣�������G����
���𰸡� ������Ӧ ![]() 2CH3CH2OH��O2
2CH3CH2OH��O2![]() 2CH3CHO��2H2O
2CH3CHO��2H2O ![]() ��3NaOH
��3NaOH![]()
![]() ��2NaBr��2H2O
��2NaBr��2H2O ![]() ��2Cu(OH)2��NaOH
��2Cu(OH)2��NaOH![]()
![]() ��Cu2O����3H2O 5
��Cu2O����3H2O 5
���������������л���A����Է�������Ϊ46����������������B����A���Ҵ���B����ȩ��B����������Ӧ���ữ������C����C�����ᡣMˮ�����������D������M�ķ���ʽ��֪D�����к���7��̼ԭ�ӡ�Dת�����ɵ�E�ܱ�������ͭ����������ɫ������F����E�����к���ȩ����������֪��Ϣ��֪Dˮ������ȩ����F�ữ����G���л���G��FeC13��Һ��ɫ�����з��ǻ����˴Ź���������4���壬��G�Ľṹ��ʽΪ![]() ������FΪ
������FΪ![]() ��EΪ
��EΪ![]() ��DΪ
��DΪ![]() �����MΪ
�����MΪ![]() ���ݴ˽����
���ݴ˽����
��⣺��1���������Ϸ�����֪B��C�ķ�Ӧ������������Ӧ��
��2��M�Ľṹ��ʽΪ![]() ��
��
��3���������Ϸ�����֪��A��B�ķ���ʽΪ2CH3CH2OH��O2![]() 2CH3CHO��2H2O����D��E�ķ���ʽΪ
2CH3CHO��2H2O����D��E�ķ���ʽΪ![]() ��3NaOH
��3NaOH![]()
![]() ��2NaBr��2H2O����E��F�ķ���ʽΪ
��2NaBr��2H2O����E��F�ķ���ʽΪ![]() ��2Cu(OH)2��NaOH
��2Cu(OH)2��NaOH![]()
![]() ��Cu2O����3H2O��
��Cu2O����3H2O��
��4����G��Ϊͬ���칹�壬������������ȡ����������FeC13��Һ��ɫ��˵�����з��ǻ�������һ��ȡ�������Ȼ�������λ�ͼ�λ�������OOCH��������ǻ���λ�����ڼ�����֣�������5�֡�

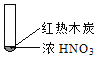
 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�����Ŀ������ʵ���о��к���ɫ����������Աȷ������ý��۲���ȷ���ǣ�������
|
|
|
�� | �� | �� |
A. �����еĺ���ɫ���壬�ƶϲ���������һ���ǻ������
B. ����ɫ���岻�ܱ�������ľ̿��Ũ��������˷�Ӧ
C. ����˵��Ũ������лӷ��ԣ����ɵĺ���ɫ����Ϊ��ԭ����
D. ������������м���CO2���ɴ�˵��ľ̿һ����Ũ���ᷢ���˷�Ӧ